Surface Tension Simple Definition Biology
Water has a high surface tension because the water molecules on the surface are pulled together by strong hydrogen bonds That means a drop of water will "want" to have the smallest possible surface area The shape that has the smallest possible area for a given volume is a sphere.
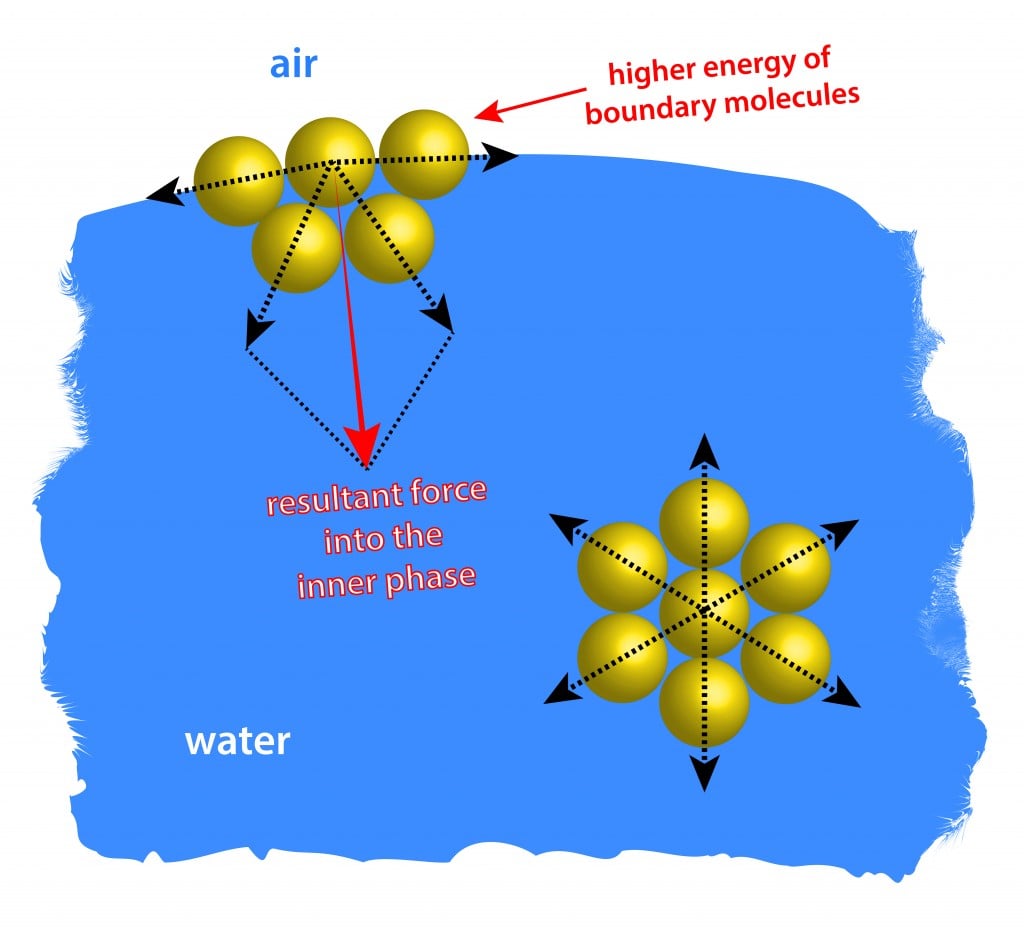
Surface tension simple definition biology. 2) Mechanical definition of surface tension Thus surface tension can manifest itself both in forms of surface energy as well as surface force (i) as an energy necessary to create surfaces Work is needed to increase the surface area eg you perform 'work' when you beat egg whites into a meringue, or you make an emulsion of water in oil while. Surface tension is a barrier formed on the surface of water caused by something called “cohesion” Liquids all have this force, a force that holds a material together Some are stronger than others (liquid mercury is a highly cohesive liquid). It has to do with the elastic property of the water surface, a phenomenon called surface tensionSubscribe http//bitl.
Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it This attraction of molecules towards one another is known as an intermolecular force. Surface Tension It is defined as the force per unit length acting at right angles to an imaginary line drawn on the free surface of the liquid When the surface is between two liquids (such as water and oil), it is known as “interface tension”. Surface tension changes in biological phenomena can determine various diseases in the human body 3 Industrial applications Surface tension is an important factor in industrial processes In all the industrial plants the R&D departments use the surface tension phenomena to improve the quality of the products.
Surface tension disinfectants Disinfectants are usually solutions of low surface tension This allow them to spread out on the cell walls of bacteria and disrupt them Soaps and detergents These help the cleaning of clothes by lowering the surface tension of the water so that it more readily soaks into pores and soiled areas. Cohesive Force Definition The force of attraction acting between the molecules of same substance is called cohesive force We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts Cohesive Force in Physics Definition, Examples – Surface Tension. Looking at surface tension from a particle perspective and a macro perspective, this vide.
Definition Of Surface Tension Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force More About Surface tension The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Surface Tension in the Lung Surface tension is the force exerted by water molecules on the surface of the lung tissue as those water molecules pull together Water (H 2 O) is a highly polar molecule, so it forms strong covalent bonds with other water molecules The force of these covalent bonds effectively creates an inward force on surfaces. Surface tension is the tendency of liquids to keep a low surface area For example, if you fill a cup of water to the top, you'll find that the water can actually rise above the lip of the cup slightly The reason it doesn't spill is that the water molecules at the surface hold together like the surface of a balloon, holding the water inside.
Some of the mechanisms they use are as follows Reducing interfacial tension Immiscible liquids are separated by their interfacial tension with each other and maintain as small a surface area touching each other as possible Some emulsifying agents decrease this interfacial tension and allow the liquids to mix seamlessly. Here are four surface tension experiments that are supersimple to set up and execute Do all four for a morning full of science & fun!. Surface Tension Surface tension, in physics, is an effect within a liquid ’ s surface layer, which causes the layer to possess characteristics similar to elastic For instance, surface tension allows insects such as a water strider to walk on water Surface tension, thus, is the result of the cohesive forces that attract water molecules to one another.
Definition Surface tension is the attractive force found in liquids which is responsible for pulling surface molecules in the rest of the liquid Further, it minimizes the surface area The attractive forces we are talking about here are because of electrostatic forces. Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin It is the high surface tension of water which allows insects to walk over it These pond skaters have long hairy legs which allow them to spread their weight over a wide area. Magic Finger Surface Tension Experiment.
•A substance that disrupts and lowers the surface tension of a liquid •Detergents contain a polar and nonpolar end When added to water, they attach to water, but do not allow waters to attach to each other, thus lowering the surface tension. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible Surface tension allows insects, to float and slide on a water surface without becoming even partly submerged Play media Surface tension and hydrophobicity interact in this attempt to cut a water droplet Play media Surface tension experimental demonstration with soap Rain water flux from a canopy Among the forces that govern drop formation surface tension, cohesion, Van der Waals force, Platea. Surfacetension phenomena are ubiquitous in organismal biology, although the subject has not yet received a unified treatment Among plants, surface tension governs the flow of xylem fluid in trees, the ejection of spores via Buller’s drop in fungi or the dispersal of seeds through the splash of raindrops (Amador et al 13).
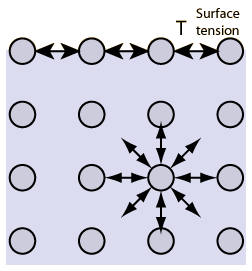
Surface tension occurs due to ionic bonding Surface tension is caused by water molecules repelling one another Surface tension allows water to support small objects if they are placed carefully on its surface Surface tension of water is weakened by hydrogen bonding. Surface tension definition, the elasticlike force existing in the surface of a body, especially a liquid, tending to minimize the area of the surface, caused by asymmetries in the intermolecular forces between surface molecules See more. This activity is a simple demonstration of surface tension When you have a container full of water, the water molecules below the surface are pulled together equally in all directions, but those on top are pulled together more tightly, as they don’t have water molecules above them, this draws them together to form a kind of ‘skin’ which.
Test your knowledge on cohesion, adhesion, and surface tension of water!. Surface Tension in the Lung Surface tension is the force exerted by water molecules on the surface of the lung tissue as those water molecules pull together Water (H 2 O) is a highly polar molecule, so it forms strong covalent bonds with other water molecules The force of these covalent bonds effectively creates an inward force on surfaces, such as lung tissue, with the effect of lowering the surface area of that surface as the tissue is pulled together. Surface Tension Definition Surface tension is a physical property equal to the amount of force per unit area necessary to expand the surface of a liquid It is the tendency of a fluid surface to occupy the smallest possible surface area Surface tension is a principal factor in capillary action The addition of substances called surfactants can reduce the surface tension of a liquid.
How do water striders walk on water?. Thus, the surface tension formula is Surface tension = (surface force)/ (length force acts) γ = F /d Over here Γ refers to the Surface tension F is the force which applies to the liquid d refers to the length where the force acts Solved Examples on Surface Tension Question You have a small piece of metal that is 1 cm long and weighs 0. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties.
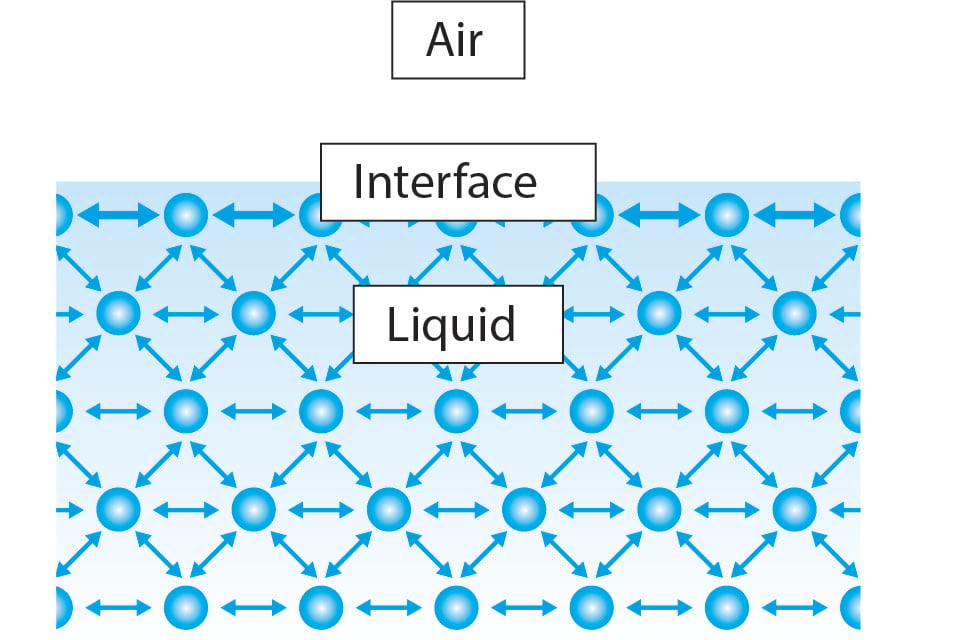
Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet This term is typically used only when the liquid surface is in contact with gas (such as the air) If the surface is between two liquids (such as water and oil), it is called "interface tension". Surface tension can be defined in terms of force or energy In terms of force Surface tension γ of a liquid is the force per unit length In the illustration on the right, the rectangular frame, composed of three unmovable sides (black) that form a "U" shape, and a fourth movable side (blue) that can slide to the right.
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is the tendency of liquids to keep a low surface area For example, if you fill a cup of water to the top, you'll find that the water can actually rise above the lip of the cup slightly The reason it doesn't spill is that the water molecules at the surface hold together like the surface of a balloon, holding the water inside. Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece See full answer below.
How does surface tension affect the surface properties of a liquid?. Units of Surface Tension Surface tension is expressed in units of force per unit length or of energy per unit area (for instance, N/m or J/m 2) The two are equivalent, but when referring to energy per unit area, people use the term “surface energy,” which is a more general term in the sense that it applies to solids as well as to liquids. Surface tension definition The "tension" in the surface is the force per unit length that must be applied parallel to the surface as to counterbalance the net inward pull Force per unit length existing at the interface between two immiscible liquids.
It happens in cell biology, where small molecules simply diffuse through the cell membrane, but larger molecules only get through by using energy see active transport The random movement of fluid molecules makes them spread out until a boundary stops them. If you're seeing this message, it means we're having trouble loading external resources on our website If you're behind a web filter, please make sure that the domains *kastaticorg and *kasandboxorg are unblocked. Surface tension is the tendency of liquids to keep a low surface area For example, if you fill a cup of water to the top, you'll find that the water can actually rise above the lip of the cup slightly The reason it doesn't spill is that the water molecules at the surface hold together like the surface of a balloon, holding the water inside.
Simple Surface Tension Experiments Water molecules like to stick together, creating surface tension where the water meets the air This force can be fun to explore!. Surfacetension phenomena are ubiquitous in organismal biology, although the subject has not yet received a unified treatment Among plants, surface tension governs the flow of xylem fluid in trees, the ejection of spores via Buller’s drop in fungi or the dispersal of seeds through the splash of raindrops (Amador et al 13). Surface tension is the amount of energy required to increase the surface of the liquid by unit area In other words, it is also the property of the liquid surface that resists force Intuitively, it keeps a barrier between foreign materials and liquid as well as this is the force that holds the liquid molecules bind together.
The surface tension of water is an important parameter for many biological or industrial processes, and roughly a factor of 3 higher than that of nonpolar liquids such as oils, which is usually attributed to hydrogen bonding and dipolar interactions. Surface energy is the work per unit area was done by the force that creates the new surface Learn the relation between surface energy and surface tension with definition, unit & formulas at BYJU'S. Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin It is the high surface tension of water which allows insects to walk over it These pond skaters have long hairy legs which allow them to spread their weight over a wide area.
Biological Importance of Surface Tension Biological Importance 1 Emulsification of fats by bile salts In the duodenum Bile salts lower the surface tension of the fat droplets that aids in digestion and absorption of lipids 2 Surface tension of plasma The plasma’s surface tension is 70 dynes/cm that is slightly lower as compared that of. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible Surface tension allows insects (eg water striders), to float and slide on a water surface without becoming even partly submerged At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the. View this answer Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece See full answer below.
Surface tension definition is the attractive force exerted upon the surface molecules of a liquid by the molecules beneath that tends to draw the surface molecules into the bulk of the liquid and makes the liquid assume the shape having the least surface area. A feeling for the scaling of surface tension The normal stress balance at a free surface must be balanced by the curvature pressure associated with the surface tension n · T · n = σ(∇ · n) (23) 1 where T = −pI µ ∇u( ) T 2 Eis the stress tensor, T 2 is the. Biology Chemistry Earth Science Health & Nutrition Nursing Physics Social Science Anthropology Psychology Sociology Science Chemistry Q&A Library A simple definition of surface tension with an example A simple definition of surface tension with an example Question Asked Jan 29, 19 7 views A simple definition of surface tension.
Biology • all small creatures live in a world dominated by surface tension Surface tension is a direct measure of this energy loss per unit area of surface If the We here simply state them for the simple case of a free surface (such as airwater, in which one of the fluids is not dynamically significant) in order to get. Surface tension definition, the elasticlike force existing in the surface of a body, especially a liquid, tending to minimize the area of the surface, caused by asymmetries in the intermolecular forces between surface molecules See more. Surface Tension A special type of cohesion is surface tension The tension on the surface of water occurs when water molecules on the outside of the system align and are held together by hydrogen bonding to create an effect similar to a net made of atoms For example, the surface tension of water allows water spiders to literally walk on water.
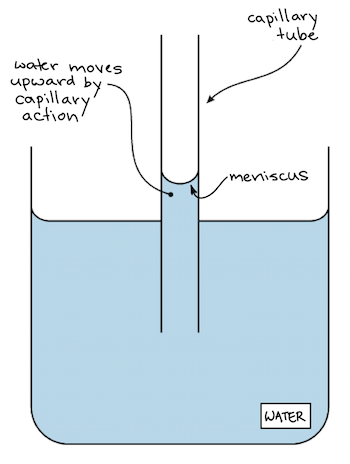
This is the upward or downward curve at the surface of a liquid in a container A meniscus occurs because of surface tension. And you have probably observed surface tension many, many, many times in your life in the form of, say, a water droplet A water droplet, it's able to have this roughly round shape because all the little water molecules on the surface of the water droplet, and here the surface might even be on the bottom of the water droplet. Surface tension is an effect where the surface of a liquid is strong The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape Some small things can float on a surface because of surface tension, even though they normally could not float.
Surface tension Definition Surface tension is a measure of how difficult it is to stretch or break the surface of a liquid Campbell Biology by JB Reece, LA Urry.

Surface Tension And Water

What Is Surface Tension

Surface Tension Definition Examples Facts Britannica
Surface Tension Simple Definition Biology のギャラリー

Surface Tension Determines Tissue Shape And Growth Kinetics Science Advances

Surface Tension Questions And Answers Topperlearning

Biol60 Cell Biology

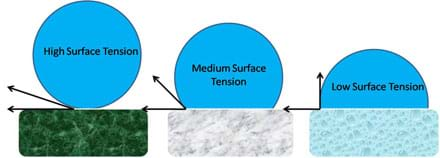
Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension
Surfactants Helping Molecules Get Along Lesson Teachengineering

Capillary Action Of Water Definition Examples Video Lesson Transcript Study Com

Solid Surface Tension Measured By A Liquid Drop Under A Solid Film Pnas

Surface Tension And Water

Water Transport In Plants Xylem Organismal Biology

Cohesive And Adhesive Properties Bioninja

Measuring Surface Interfacial Tension Ata Scientific
Surface Tension

Surface Tension Wikipedia

The Science Of New Wash Science Physical Properties Phase Rule

Surface Tension Definition Examples Facts Britannica

Xylem Definition And Examples Biology Online Dictionary
Surface Tension Of Water Why Is It So High

Surface Tension Definition Examples Facts Britannica
What Is Surface Tension And Its Applications Quora

Surface Tension

Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
Surface Tension Facts For Kids

Lesson 10 Properties Of Water

Surface Tension Determines Tissue Shape And Growth Kinetics Science Advances

Water And Its Structure
Q Tbn And9gcspsocbabgtgsjjl Okv27x0clb5b Rrhbermhi1nov5bdemmgn Usqp Cau

Pdf Surface Tension Of The Most Popular Models Of Water By Using The Test Area Simulation Method

Surface Tension Wikipedia
Cohesion And Adhesion In Liquids Surface Tension And Capillary Action Physics

Pdf Surface Tension
/water-dropping-into-glass-108190099-5884c9413df78c2ccd007c7e.jpg)
Cohesion Definition And Examples In Chemistry

What Is Surface Tension

Biol60 Cell Biology

Surface Tension Of Seawater Journal Of Physical And Chemical Reference Data Vol 43 No 4

Super Simple Surface Tension Science Experiments For Kids

Surface Tension Dominates Insect Flight On Fluid Interfaces Journal Of Experimental Biology

How Many Drops Of Water Can Fit On A Penny Surface Tension Experiment
Tension Force Definition Formula Examples And Newton S Law Of Motion

Surface Tension Questions And Answers Topperlearning

Water Boundless Biology
Water Transport In Plants Xylem Organismal Biology
Core Ac Uk Download Pdf Pdf

Dynamic Surface Tension Measurements As General Approach To The Analysis Of Animal Blood Plasma And Serum Sciencedirect
Q Tbn And9gcraa9qoyuwkyxb Zb9nkjkbouqk8yxrvxb8eqa2rrurfcjnr8 W Usqp Cau

Surface Tension Video Chemistry Of Life Khan Academy
Surface Tension
Cohesion And Adhesion Of Water Article Khan Academy

What Is Surface Tension Cool Science Experiment
/Surface-Tension-58c6c2365f9b58af5c534f71.jpg)
What Is Surface Tension Definition And Experiments

Capillary Action Wikipedia

Surface Tension And Adhesion Video Khan Academy

Surface Tension Determines Tissue Shape And Growth Kinetics Science Advances
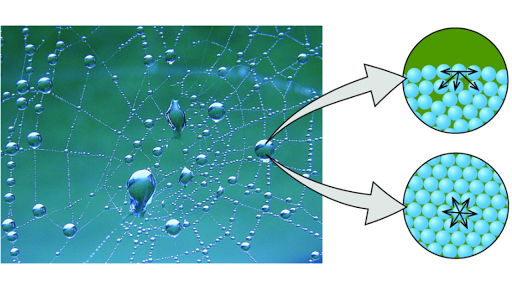
Cohesion And Adhesion Of Water Article Khan Academy

Surface Tension Definition Causes Measurement Formula Video Lesson Transcript Study Com

Pdf Surface Tension From Fundamental Principles To Applications In Liquids And In Solids

2 2 Water Concepts Of Biology 1st Canadian Edition
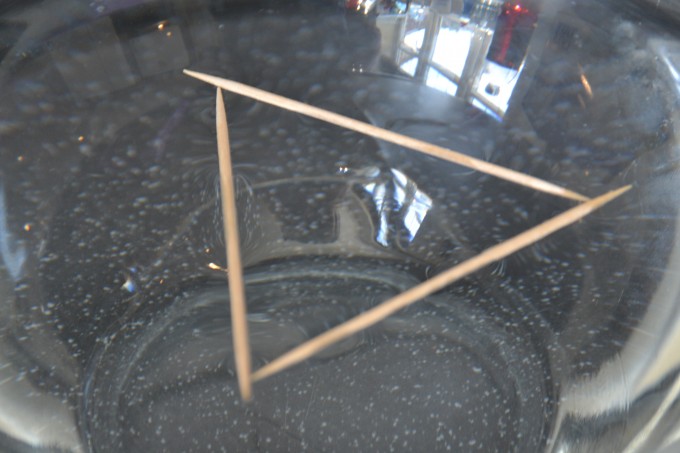
Super Simple Surface Tension Science Experiments For Kids

Surface Tension Dominates Insect Flight On Fluid Interfaces Journal Of Experimental Biology
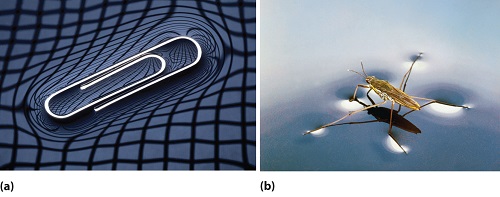
Surface Tension Chemistry Libretexts

Universal Solvent Definition And Characteristics Biology Dictionary

Surface Tension Definition Explanation Examples And Significance

Methods Of Surface Tension Measurement Ata Scientific

Plant Xylem Hydraulics What We Understand Current Research And Future Challenges Venturas 17 Journal Of Integrative Plant Biology Wiley Online Library

Measuring Bilayer Surface Energy And Curvature In Asymmetric Droplet Interface Bilayers Journal Of The Royal Society Interface
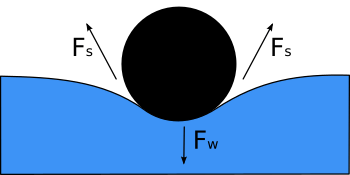
Surface Tension Facts For Kids

Surface Tension Of Deionized Water Experiment
:max_bytes(150000):strip_icc()/what-are-alveoli-2249043-01-94dfddd4dfe9488b8056d586824c7c36.png)
Alveoli Structure Function And Disorders Of The Lungs

What Is Surface Tension Definition Si Unit Formula Dimension And Examples
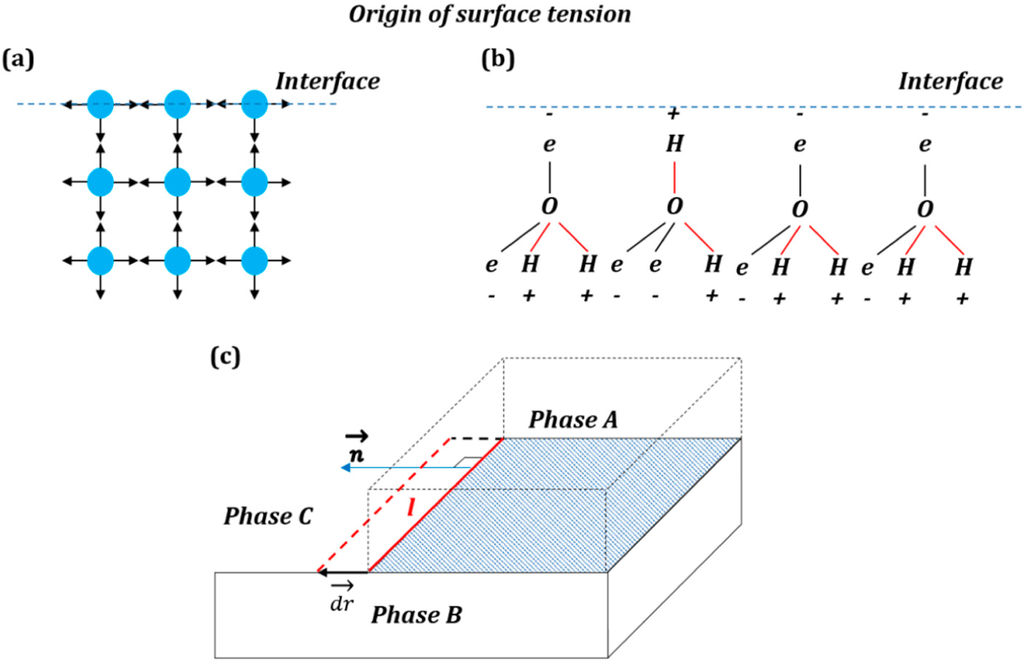
Entropy Free Full Text Geometric Interpretation Of Surface Tension Equilibrium In Superhydrophobic Systems Html

Build A Raft Powered By Surface Tension Science Project

Surface Tension Definition Explanation Examples And Significance
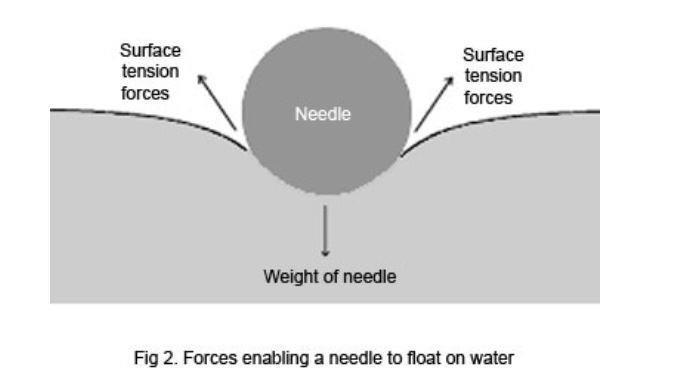
Detergents Soaps And Surface Tension Experiment Rsc Education
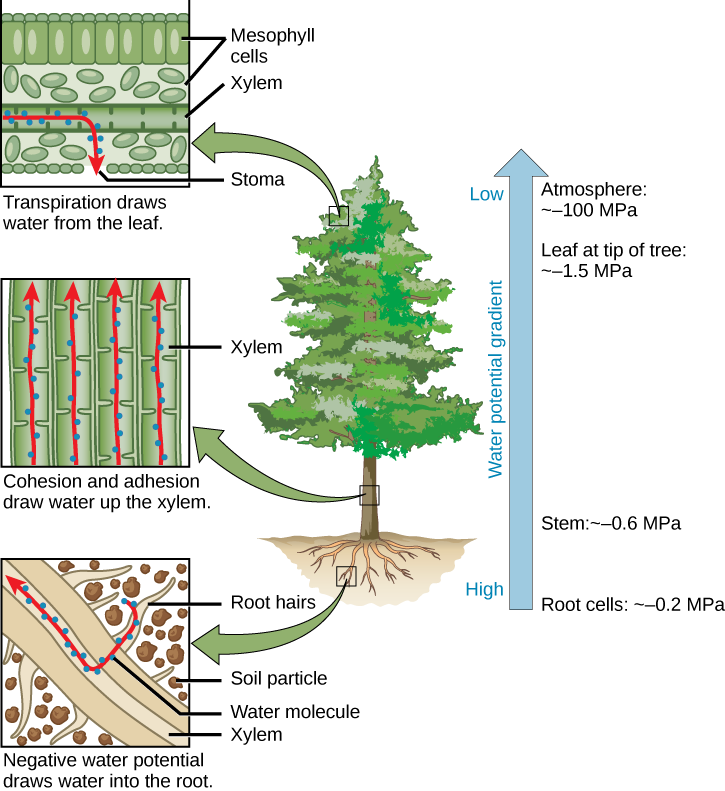
Transpiration Biology For Majors Ii

Measuring Bilayer Surface Energy And Curvature In Asymmetric Droplet Interface Bilayers Journal Of The Royal Society Interface

Surface Tension And Water

To Determine The Surface Tension Of Water By Capillary Rise Method Learn Cbse

10 Surface Tension Ideas Surface Tension Tension Surface
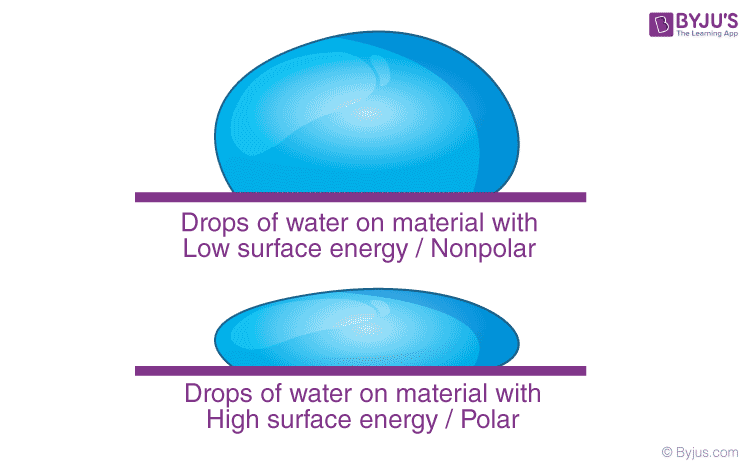
What Is Surface Energy Definition Formula Units Equation
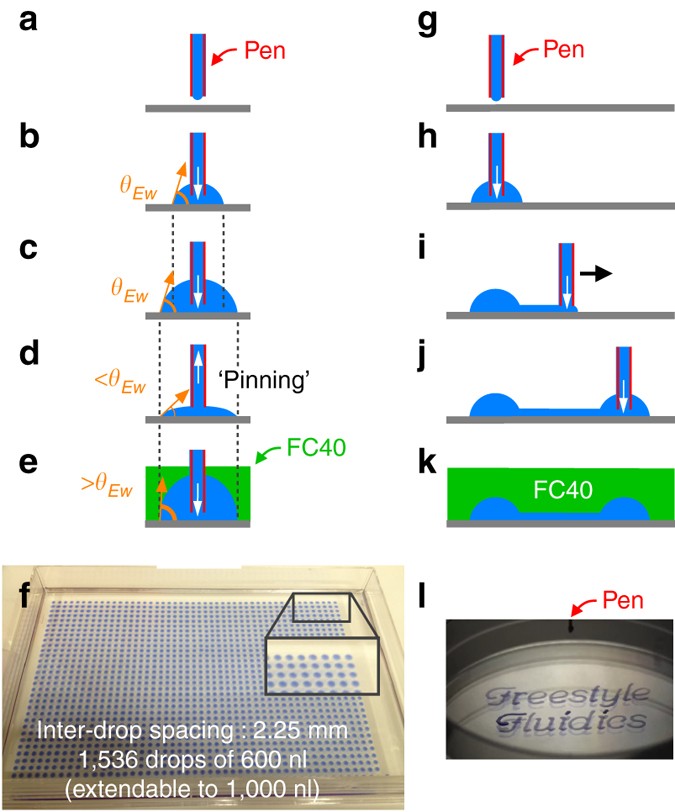
Microfluidics With Fluid Walls Nature Communications

Dynamic Surface Tension Measurements As General Approach To The Analysis Of Animal Blood Plasma And Serum Sciencedirect

2 2 Water Concepts Of Biology 1st Canadian Edition

Use Surface Tension To Make Pepper Dance Scientific American

The Surface Tension Of Surfactant Containing Finite Volume Droplets Pnas

Surface Tension And Surfactant Fluid Mechanics Lesson 12 Youtube

Defining Useful Molecules Biopolyverse

Surface Tension And Water

Surface Tension Formula Definition Equations Examples
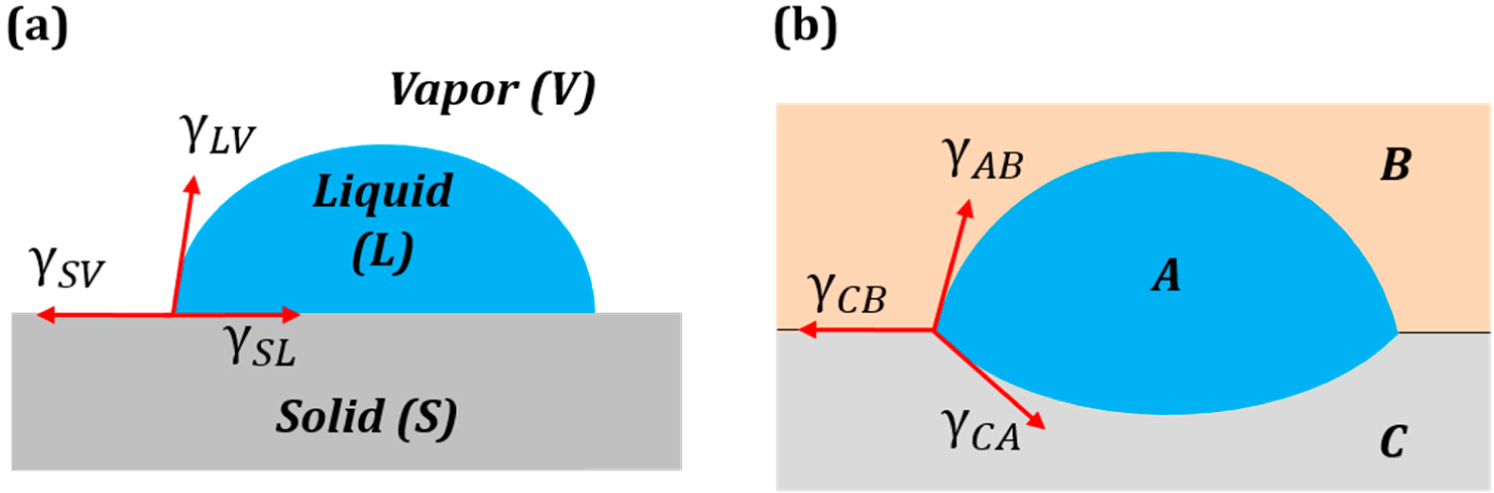
Entropy Free Full Text Geometric Interpretation Of Surface Tension Equilibrium In Superhydrophobic Systems Html
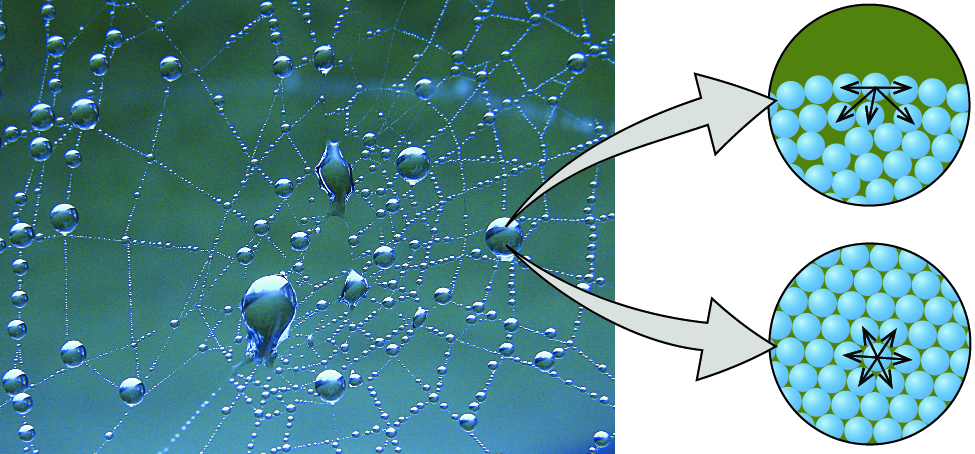
Cohesion And Adhesion Of Water Article Khan Academy

Surface Tension Facts For Kids
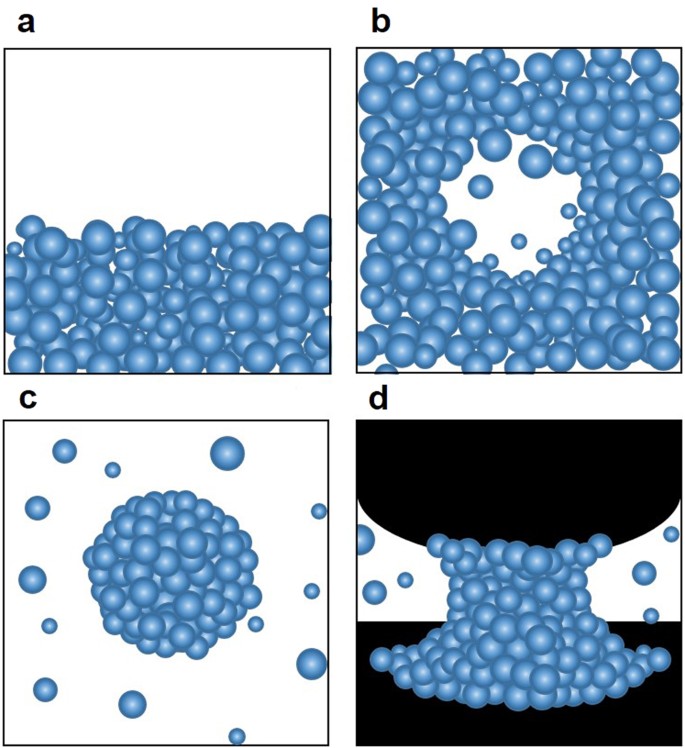
Adhesive Force Measurement Of Steady State Water Nano Meniscus Effective Surface Tension At Nanoscale Scientific Reports
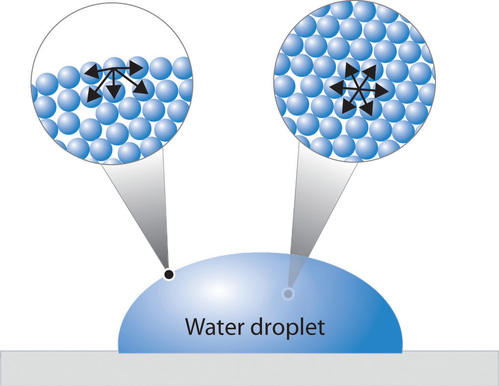
Surface Tension Chemistry Libretexts

Surface Tension Wikipedia

Surface Tension Definition Formula Examples Capillary Effect
1
Q Tbn And9gcrtwstk2chkikbjkncc Wvnowmcu1ciuzwui92axpa7pwuibidk Usqp Cau

Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension

Surface Tension And Water
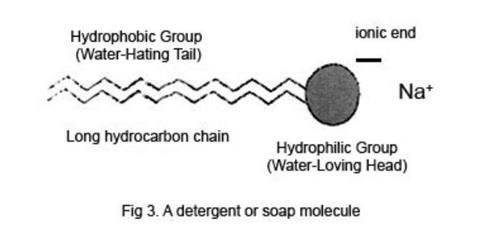
Detergents Soaps And Surface Tension Experiment Rsc Education

Capillary Action In Plants Definition Examples Biology Class Video Study Com
/139802493-56a12f615f9b58b7d0bcde91.jpg)
Surface Tension Definition In Chemistry