Surface Tension Simple Definition In Physics
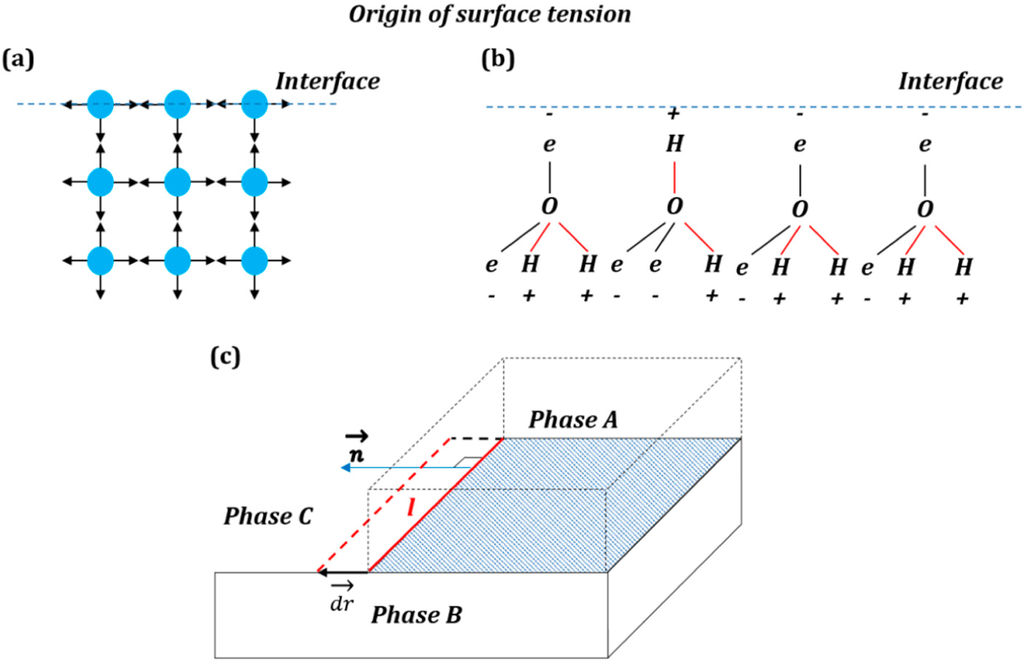
51 Basic physics of surface tension Although surface tension may be taken as a primary phenomenological concept in continuum physics, it is nevertheless instructive first to make a simple molecular model which captures the essential features of the phenomenon and even allows us to make an estimate of its mag.

Surface tension simple definition in physics. Surface tension can be defined in terms of force or energy In terms of force Surface tension γ of a liquid is the force per unit length In the illustration on the right, the rectangular frame, composed of three unmovable sides (black) that form a "U" shape, and a fourth movable side (blue) that can slide to the right. Definition of surface tension the attractive force exerted upon the surface molecules of a liquid by the molecules beneath that tends to draw the surface molecules. When someone relaxes gently on water, it creates a small depression on the water's surface Surface tension and buoyancy both work together to keep the person or object a float Surface tension keeps the person or object from falling into the water;.
When the brick is getting an upward acceleration, the Tension Formula Physics is expressed as T (Tension in A String Formula) = mg ma = 10 × 98 10 × 3 = 128 N Surface Tension Equation Surface tension is defined as a phenomenon that happens when a phase has made the interaction with the surface of a liquid The other phase can also be. Surface tension tends to reduce with increasing temperature As a liquid heats up, the molecules in it speed up, which tends to break the bonds produced by cohesive forces Table 2 shows the effect of temperature on the surface tension of water The colder the water, the higher the surface tension. Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with itThe molecules in a drop of water, for example, attract each other weakly Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules.
Surface tension changes in biological phenomena can determine various diseases in the human body 3 Industrial applications Surface tension is an important factor in industrial processes In all the industrial plants the R&D departments use the surface tension phenomena to improve the quality of the products. You might have heard the term of surface tension in various chapters if you are a physics student Imagine a water droplet on a leaf after a rain shower The water creates stunning round droplets All these things happen due to the surface tension of water Thus, we will learn surface tension formula in this article. Thus we can define surface tension as the work required to create unit area of new surface The conditions under which this work is done have to be carefully defined in any precise definition, and, from a thermodynamical point of view, the strict definition is the increase in the Gibbs free energy per unit area of new surface created under.
The surface tension of aq solns of simple inorg electrolytes (36 in total) were measured by the max bubble pressure method as a function of electrolyte concn ≤ 1 M In most cases the surface tension increased, however in a minority of cases, certain combinations of cations and anions had a negligible effect or decreased surface tension. We consider a liquidvapor interface in thermal equilibrium The tangential component of the pressure tensor is supposed to depend explicitly upon the position and the density profile Under this hypothesis the mechanical definition of surface tension becomes a finite summation ofN1 terms related directly to the local compressibility When the inhomogeneous compressibility equation is. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane This phenomenon can be observed in the nearly spherical.
Surface Tension and Capillary Effect. Surface tension is the elastic tendency of a fluid surface which makes it acquire the least surface area possibleThe cohesive forces between liquid molecules are responsible for the phenomenon Some notable applications of surface tension are. Notes for Surface Tension and Viscosity chapter of class 11 physics Dronstudy provides free comprehensive chapterwise class 11 physics notes with proper images & diagram SURFACE TENSION When a small quantity of water is poured on a clean glass plate, it spreads in all directions in the form of a thin film But when.
Wall Tension Pascal's principle requires that the pressure is everywhere the same inside the balloon at equilibrium But examination immediately reveals that there are great differences in wall tension on different parts of the balloon The variation is described by Laplace's Law. The surface energy of a liquid (liquidgaseous interface) can be determined with a simple model experiment Inside a Ushaped wire frame with a movable hoop at the open end a thin liquid film (eg soap solution) is spanned (see figure 3)The surface forces of the liquid film and the gravitational force of the movable hoop are in equilibrium. But buoyancy pulls up the person or object Surface tension is made by the molecules in the water.
When someone relaxes gently on water, it creates a small depression on the water's surface Surface tension and buoyancy both work together to keep the person or object a float Surface tension keeps the person or object from falling into the water;. Surface tension can be defined in terms of force or energy In terms of force Surface tension γ of a liquid is the force per unit length In the illustration on the right, the rectangular frame, composed of three unmovable sides (black) that form a "U" shape, and a fourth movable side (blue) that can slide to the right. Surface tension is the tensile force acting on either side of any imaginary line projected on the surface of a liquidit can be also defined by energy per unit area Surface tension develop only at the surface of liquid not inside itbecause of.
“Surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to. Surface tension is a critical factor in the marvel of capillarity Surface tension has the component of force per unit length, or of energy per unit area These two are comparable, however when alluding to energy per unit of the region, usually to utilize the term surface energy, which is a progressively broad term as it applies likewise to solids. Surface tension in Physics topic From Longman Dictionary of Contemporary English surface tension ˌsurface ˈtension noun uncountable HP the way the molecule s in the surface of a liquid stick together so that the surface is held together Examples from the Corpus surface tension • The jersey, which was extra small, had shoulder straps that were hanging on by surface tension and willpower.
How do water striders walk on water?. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface area These attractive forces are due to electrostatic forces. Tension can be defined as an actionreaction pair of forces acting at each end of the said elements While considering a rope, the tension force is felt by every section of the rope in both the directions, apart from the endpoints The endpoints experience tension on one side and the force from the weight attached.
Surface tension in Physics topic From Longman Dictionary of Contemporary English surface tension ˌsurface ˈtension noun uncountable HP the way the molecule s in the surface of a liquid stick together so that the surface is held together Examples from the Corpus surface tension • The jersey, which was extra small, had shoulder straps that were hanging on by surface tension and willpower. It is important to understand that tension is a pull in a connector In contrast, consider the phrase “You can’t push a rope” The tension force pulls outward along the two ends of a rope Consider a person holding a mass on a rope as shown in Figure 415. Surface tension tends to reduce with increasing temperature As a liquid heats up, the molecules in it speed up, which tends to break the bonds produced by cohesive forces Table 2 shows the effect of temperature on the surface tension of water The colder the water, the higher the surface tension.
The surface then appears to act like an extremely thin membrane, and the small volume of water that makes up a drop assumes the shape of a sphere, held constant when an equilibrium between the internal pressure and that due to surface tension is reached Because of surface tension, various small insects are able to skate across the surface of a. Surface Tension The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid The directed contracting force which attracts. Surface tension is also defined as the tensile forces acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids such that the contact surface behaves like a membrane under tension.
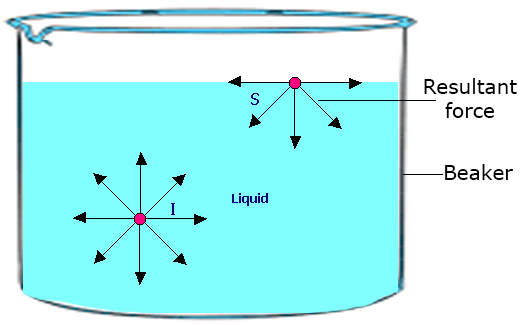
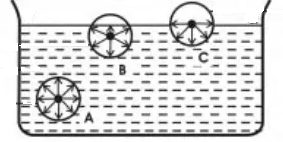
Surface Tension In Physics, the tension of the surface film of a liquid because of the attraction of the surface particles by the bulk of the liquid, which tries to minimize surface area is called surface tension When the surface of the liquid is strong enough, then surface tension is applicable It is strong enough to hold weight. Surface tension is an effect where the surface of a liquid is strong The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in. This activity is a simple demonstration of surface tension When you have a container full of water, the water molecules below the surface are pulled together equally in all directions, but those on top are pulled together more tightly, as they don’t have water molecules above them, this draws them together to form a kind of ‘skin’ which.
Surface tension is an effect where the surface of a liquid is strong The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape Some small things can float on a surface because of surface tension, even though they normally could not float. Surface tension definition, the elasticlike force existing in the surface of a body, especially a liquid, tending to minimize the area of the surface, caused by asymmetries in the intermolecular forces between surface molecules See more. 51 Basic physics of surface tension Although surface tension may be taken as a primary phenomenological concept in continuum physics, it is nevertheless instructive first to make a simple molecular model which captures the essential features of the phenomenon and even allows us to make an estimate of its mag.
Surface tension tends to reduce with increasing temperature As a liquid heats up, the molecules in it speed up, which tends to break the bonds produced by cohesive forces Table 2 shows the effect of temperature on the surface tension of water The colder the water, the higher the surface tension. Inks and paints (see Figure 6) are everyday examples in which lowering surface tension is useful in making liquids spread Using surfactants to lower surface tension is also used in the oil industry Surface tension causes oil to become trapped in the pores of the containing rock due to a phenomenon called capillary action. Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible Surface tension allows insects (eg water striders), to float and.
When the brick is getting an upward acceleration, the Tension Formula Physics is expressed as T (Tension in A String Formula) = mg ma = 10 × 98 10 × 3 = 128 N Surface Tension Equation Surface tension is defined as a phenomenon that happens when a phase has made the interaction with the surface of a liquid The other phase can also be. Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet This term is typically used. Surface Tension The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface This forms a surface "film" which makes it more difficult to move an object through the.
Surface tension disinfectants Disinfectants are usually solutions of low surface tension This allow them to spread out on the cell walls of bacteria and disrupt them Soaps and detergents These help the cleaning of clothes by lowering the surface tension of the water so that it more readily soaks into pores and soiled areas. It has to do with the elastic property of the water surface, a phenomenon called surface tensionSubscribe http//bitl. When someone relaxes gently on water, it creates a small depression on the water's surface Surface tension and buoyancy both work together to keep the person or object a float Surface tension keeps the person or object from falling into the water;.
Surface Tension Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force It is shown, for example, in the floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects (eg, water striders) to run on water’s surface. Mathematically, the internal surface forces are represented by surface tension, defined as the normal force per unit of length This is quite analogous to bulk tension. Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece.
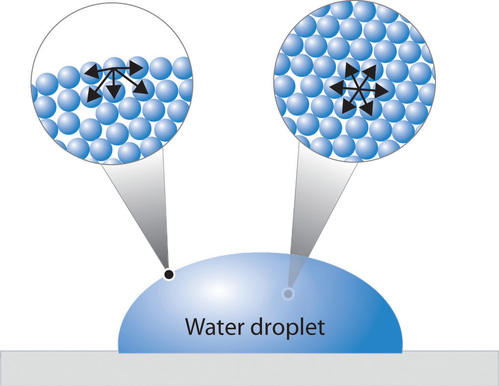
And you have probably observed surface tension many, many, many times in your life in the form of, say, a water droplet A water droplet, it's able to have this roughly round shape because all the little water molecules on the surface of the water droplet, and here the surface might even be on the bottom of the water droplet. Surface tension definition a property of liquids caused by intermolecular forces near the surface leading to the Meaning, pronunciation, translations and examples. So making more surface requires pulling molecules apart It takes more energy to make more surface, so the molecules pull together to reduce the surface area That pull is called the surface tension A freefloating drop forms a sphere because that's the way the molecules can pull together to make the least surface, for a given total volume.
Surface tension is also defined as the tensile forces acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids such that the contact surface behaves like a membrane under tension. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension The molecules at the surface do not have other like molecules. When the brick is getting an upward acceleration, the Tension Formula Physics is expressed as T (Tension in A String Formula) = mg ma = 10 × 98 10 × 3 = 128 N Surface Tension Equation Surface tension is defined as a phenomenon that happens when a phase has made the interaction with the surface of a liquid The other phase can also be.
What is Surface TensionSurface tension is the property of the free surface of a liquid at rest behave like a stretched membrane in order to acquire minimum s. When the brick is getting an upward acceleration, the Tension Formula Physics is expressed as T (Tension in A String Formula) = mg ma = 10 × 98 10 × 3 = 128 N Surface Tension Equation Surface tension is defined as a phenomenon that happens when a phase has made the interaction with the surface of a liquid The other phase can also be. Bouncing coalescence coalescence cascade drops insects liquid Locomotion milk physics science slow motion surface tension technology water water strider TKSST is an unprecedented collection of 4,500 kidfriendly videos, curated for teachers and parents who want to share smarter, more meaningful media in the classroom and at home.
Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface area These attractive forces are due to electrostatic forces. In physics, surface tension is a force present within the surface layer of a liquid that causes the layer to behave as an elastic sheet It is the force that supports insects that walk on water, for example Surface tension is caused by the attraction between the molecules of the liquid In the bulk of the liquid each molecule is pulled equally in all directions by neighbouring molecules. Define surface tension surface tension synonyms, surface tension pronunciation, surface tension translation, English dictionary definition of surface tension n 1.
Since surface tension is determined at a molecular level, any change to the component liquids, surfactants, fuels or compounds in a liquid would result in a change in the surface tension If the surface tension of a perfectly pure composition is known, any variation from that would reveal some level of contamination. Surface tension definition a property of liquids caused by intermolecular forces near the surface leading to the Meaning, pronunciation, translations and examples.

Rame Hart Instrument Co Monthly Newsletter
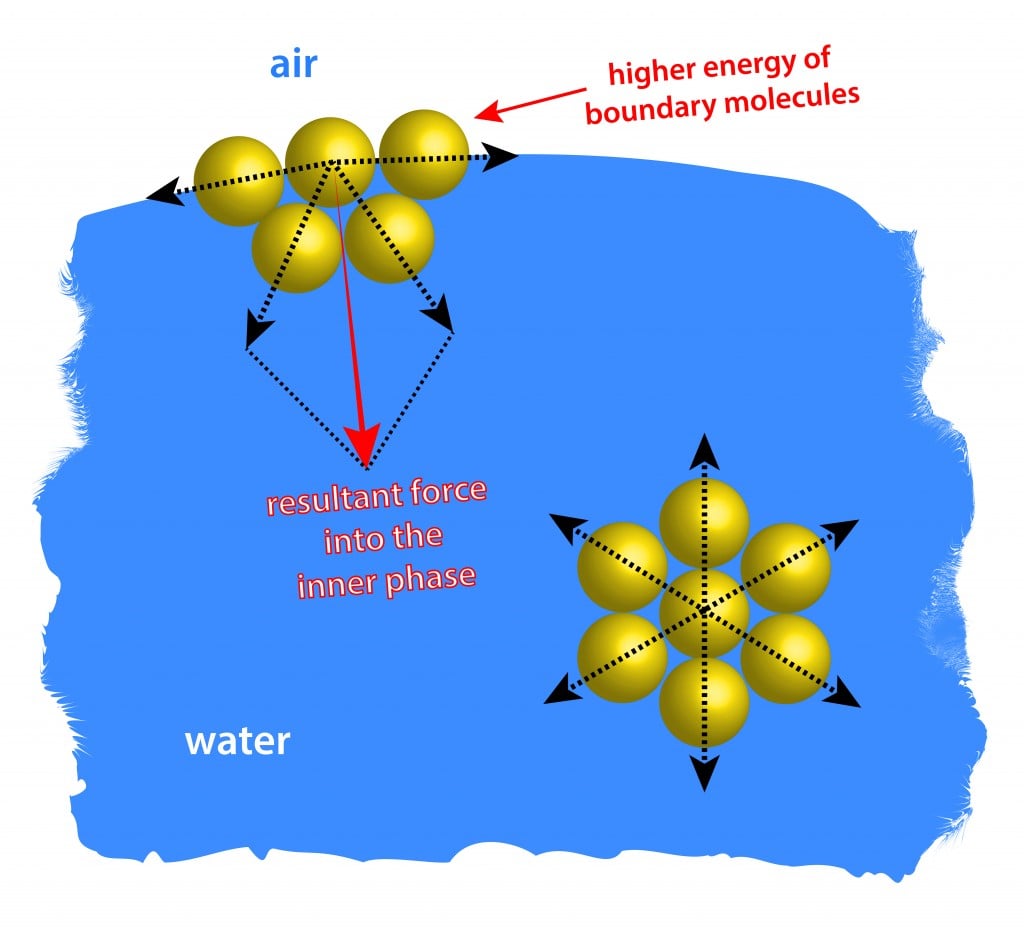
Surface Tension Definition Explanation Examples And Significance

Pdf Surface Tension
Surface Tension Simple Definition In Physics のギャラリー
:max_bytes(150000):strip_icc()/waterdrops-splashing-on-water-surface-522937305-582494ed5f9b58d5b15af89b.jpg)
Liquid Definition In Chemistry

Surface Tension And Water

What Is The Difference Between Surface Tension And Surface Energy Quora

Surface Tension Definition Examples Facts Britannica

What Is Surface Tension Properties Of Liquids Basic Physics Msbte Ekeeda Com Youtube
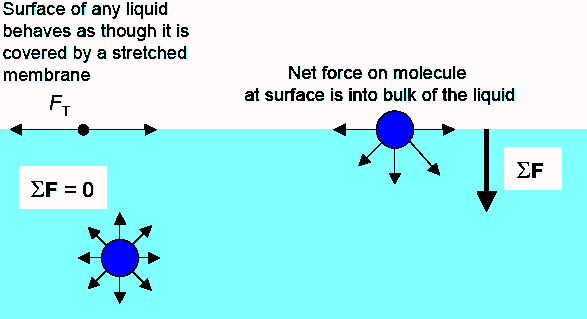
Surface Tension

Surface Tension Definition Examples Facts Britannica

11 8 Cohesion And Adhesion In Liquids Surface Tension And Capillary Action College Physics Openstax
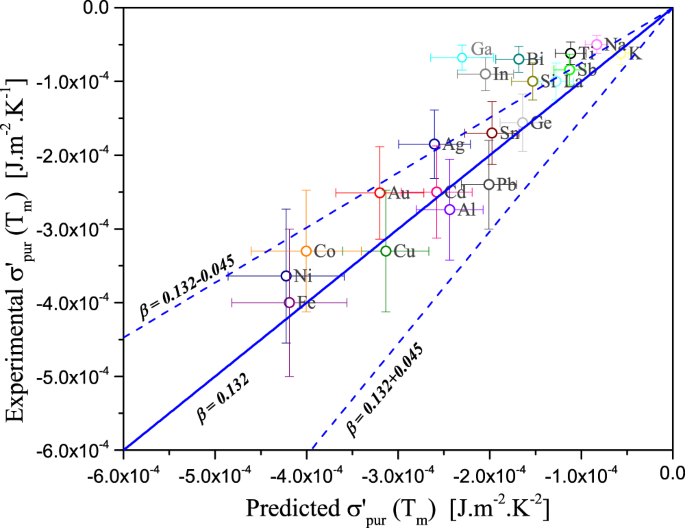
Temperature And Oxygen Adsorption Coupling Effects Upon The Surface Tension Of Liquid Metals Scientific Reports

Surface Tension Facts For Kids
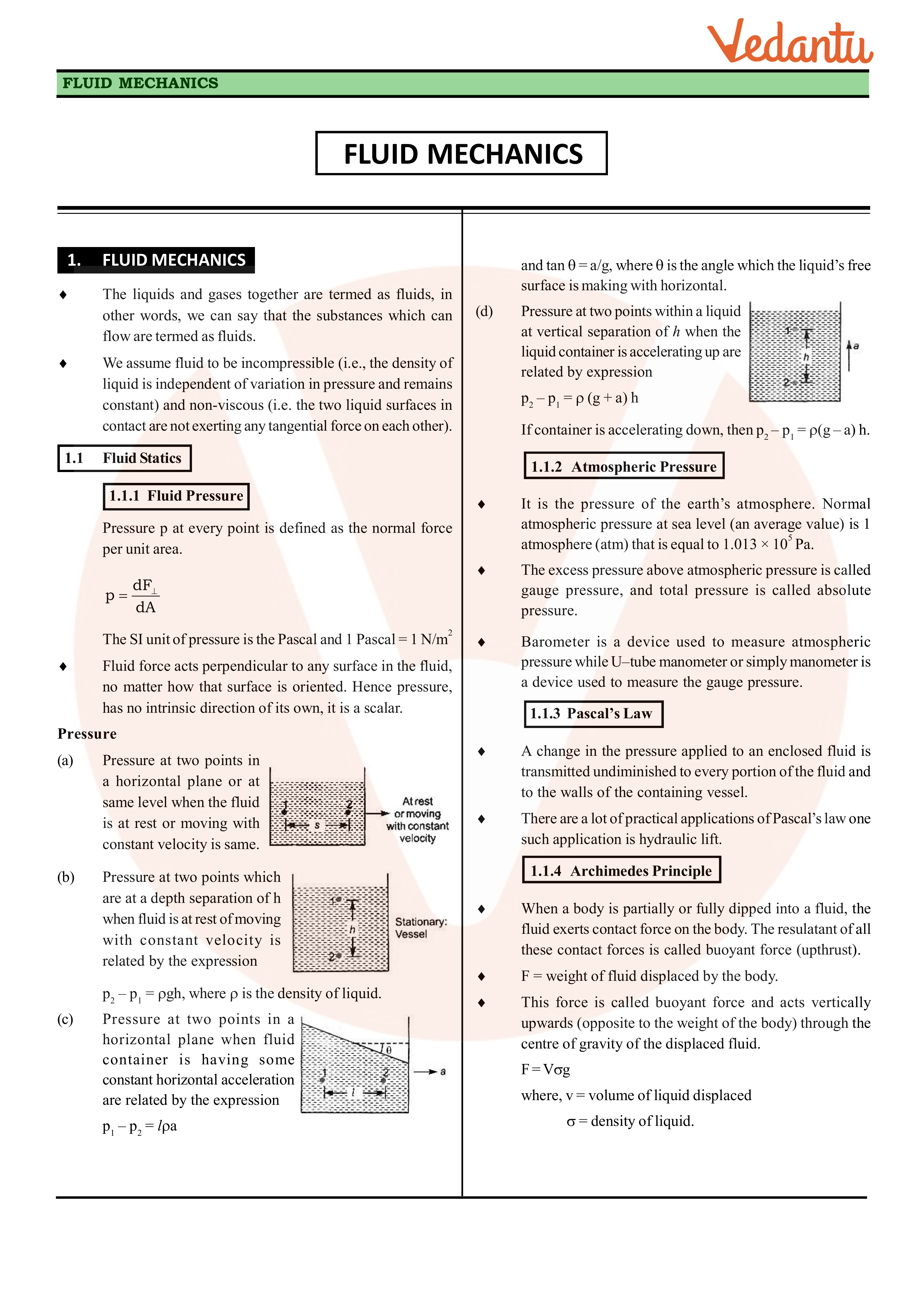
Class 11 Physics Revision Notes For Chapter 10 Mechanical Properties Of Fluids
What Is The Difference Between Surface Tension And Surface Energy Quora

What Is Surface Tension Definition Si Unit Formula Dimension And Examples

Surface And Interfacial Tension

Surface Tension

Surface Tension Simple English Wikipedia The Free Encyclopedia
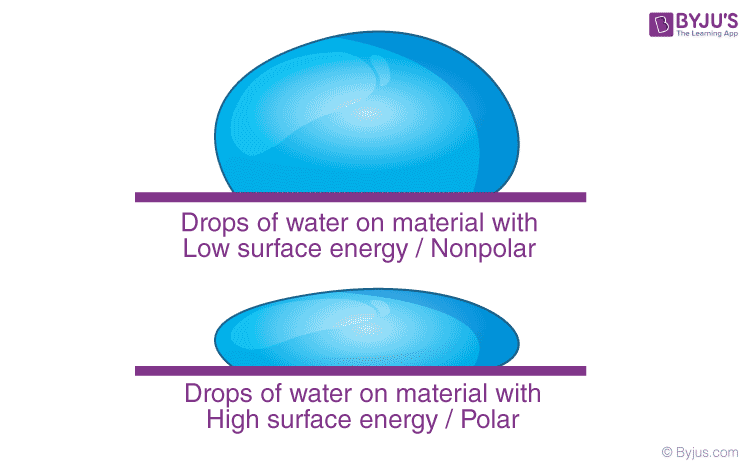
What Is Surface Energy Definition Formula Units Equation

Pdf Molecular Theory Of Surface Tension
:max_bytes(150000):strip_icc()/AZJFaceShot-56a72b155f9b58b7d0e783fa.jpg)
What Is Surface Tension Definition And Experiments

Surface Tension Physics Made Easy Facebook

Try This Walking On Water With Science Science News For Students
What Is The Difference Between Surface Tension And Surface Energy Quora
Arxiv Org Pdf 1306 4566

Hydrophobic And Hydrophilic Effects Coat Notes

The Meaning Of Surface Tension And Its Practical Applications Science Struck

Surface Tension And Water

Molecular Theory Of Surface Tension

Surface Tension Simple English Wikipedia The Free Encyclopedia

Teach Your Students About Surface Tension With This Free Lesson Plan There Are Two Key Conce Science Teacher Resources Middle School Chemistry Surface Tension

Physics Free Notes Surface Tension Theory By Atc

Measuring The Surface Tension Of Water Science Project

3 Ways To Measure Surface Tension Wikihow

Surface Tension Video Chemistry Of Life Khan Academy
Q Tbn And9gcs2dewmrnfkwbvqtefgn G5t8jzceppfzaciyhuwup9bsvrrlkl Usqp Cau

Surface Tension
Surface Tension

Surface Tension And Water

Surface Tension
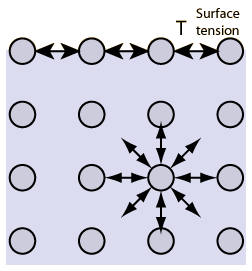
Surface Tension

Water Droplets Make An Impact Physics World

Surface Tension Of Water Alcohol Mixtures From Monte Carlo Simulations The Journal Of Chemical Physics Vol 134 No 4
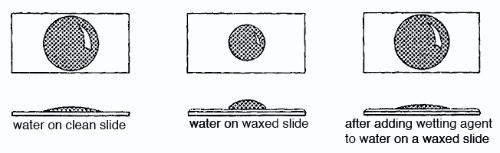
More Surface Tension Effects Iopspark

Definition Of Surface Tension Properties Of Fluid Fluid Mechanics Youtube

Surface Tension Questions And Answers Topperlearning

Surface Tension Wikipedia

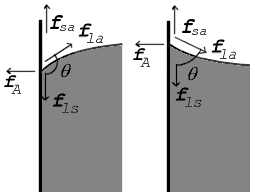
Surface Tension Contact Angle Drop Liquid Curvature
Fluids At Rest And In Motion Classhall Com
Entropy Free Full Text Geometric Interpretation Of Surface Tension Equilibrium In Superhydrophobic Systems Html

Surface Tension And Water

Capillarity Measuring Surface Tension Lesson Teachengineering
Surface Tension Simple English Wikipedia The Free Encyclopedia
Surface Tension The Concept Its Characteristics And Factors Affecting It

Surface Tension Definition Causes Measurement Formula Video Lesson Transcript Study Com
Q Tbn And9gcq5epwbuxoeeupidtn2kot Bq4ybg2lv1wohclyz Qarizayl Usqp Cau
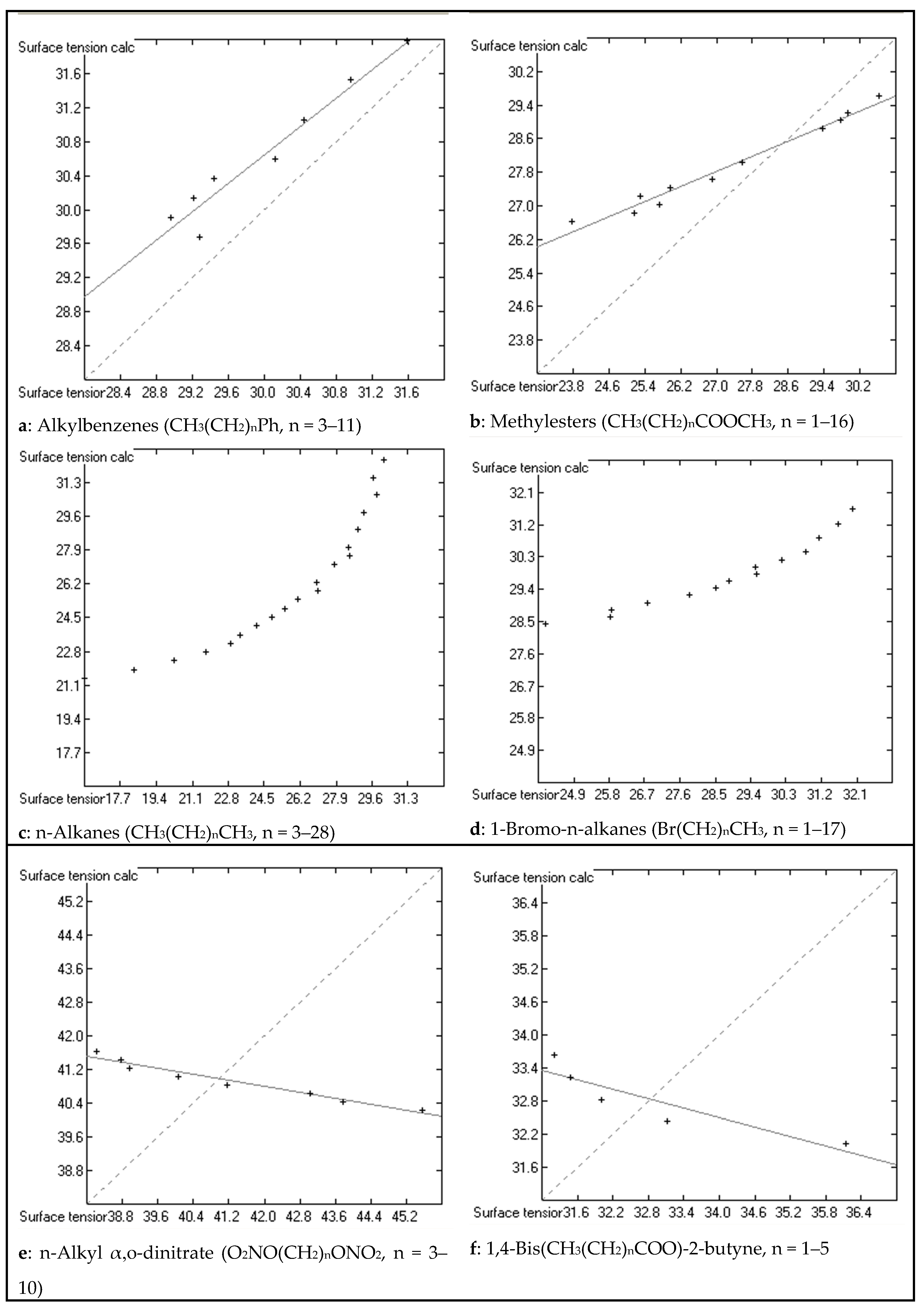
Molecules Free Full Text Calculation Of The Surface Tension Of Ordinary Organic And Ionic Liquids By Means Of A Generally Applicable Computer Algorithm Based On The Group Additivity Method Html
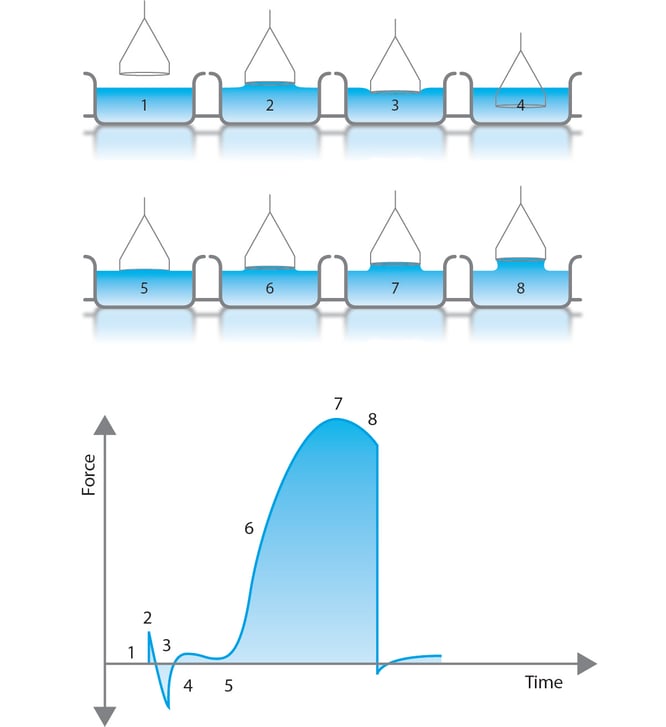
3 Ways To Measure Surface Tension

Important Questions For Cbse Class 11 Physics Chapter 10 Mechanical Properties Of Fluids

Physics Free Notes Surface Tension Theory By Atc

Surface Tension Facts For Kids Kidzsearch Com

What Is Surface Tension
Www Jstor Org Stable
1

Surface Tension Force An Overview Sciencedirect Topics
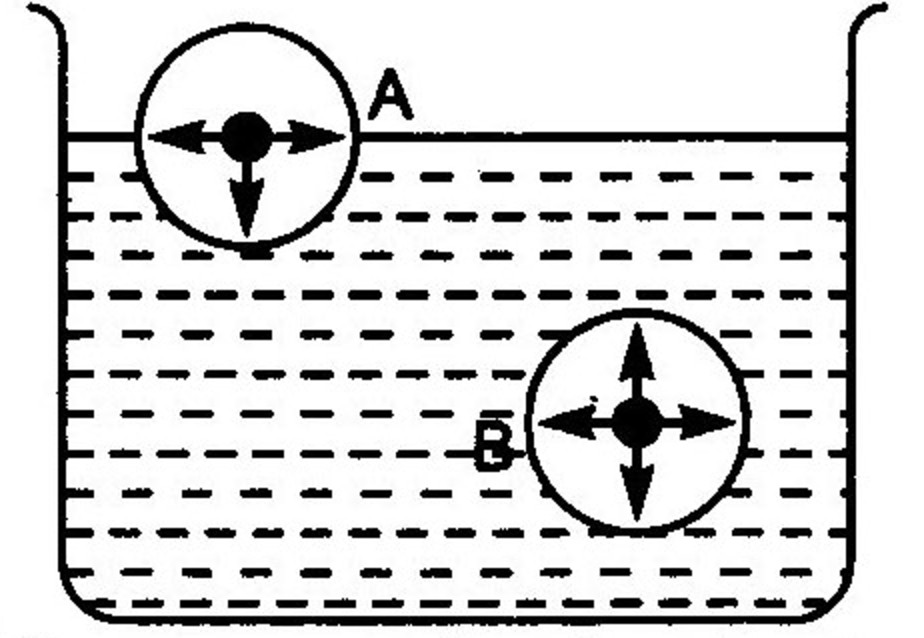
Surface Tension Chemistry Class 11 States Of Matter

Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube
Q Tbn And9gct71stnvkrj1pmdrcofq2pul Ioy0jws7hcuereabdenmugt7 Usqp Cau

Pdf Measurement Of Surface Interfacial Tension As A Function Of Temperature Using Pendant Drop Images
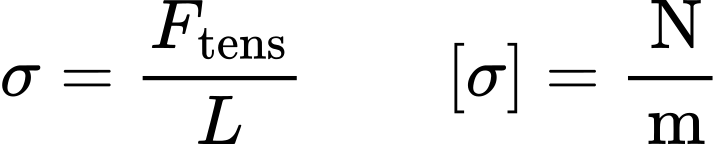
Surface Tension Surface Energy Dataphysics Instruments
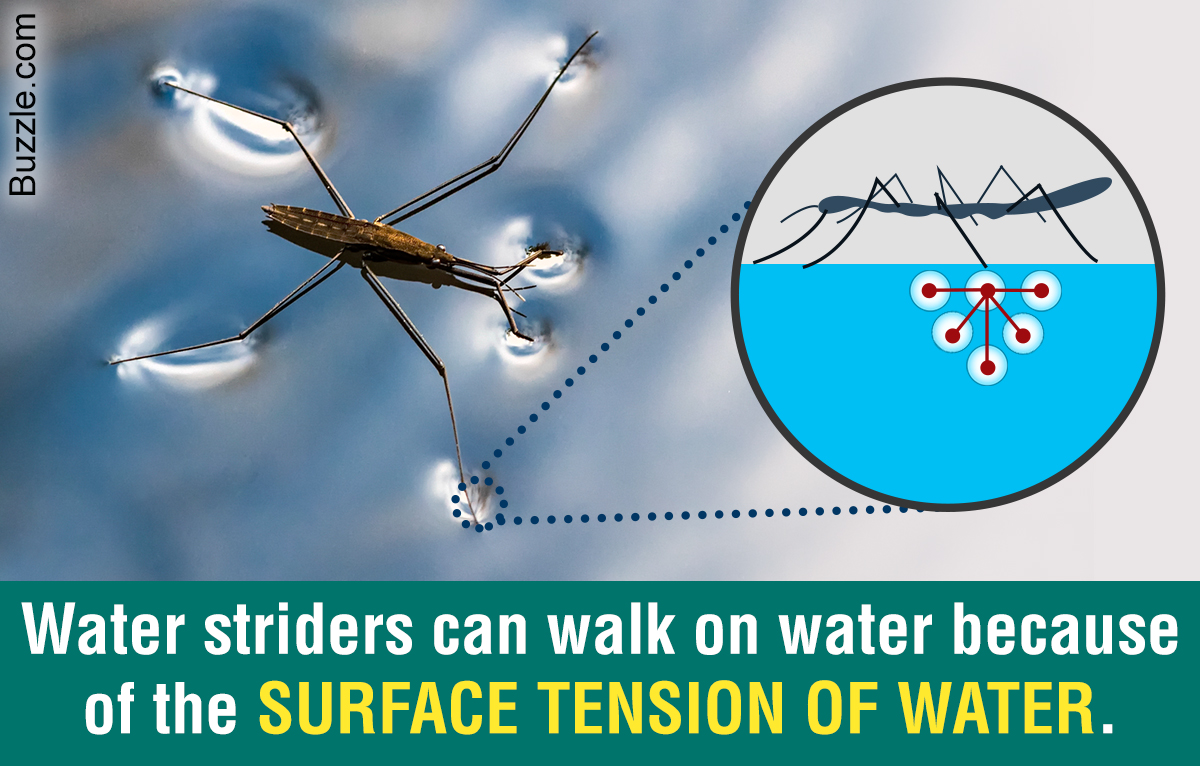
The Meaning Of Surface Tension And Its Practical Applications Science Struck
What Is The Dimensional Formula For Surface Tension Quora

Surface Tension Ideas Surface Tension Tension Science Experiments
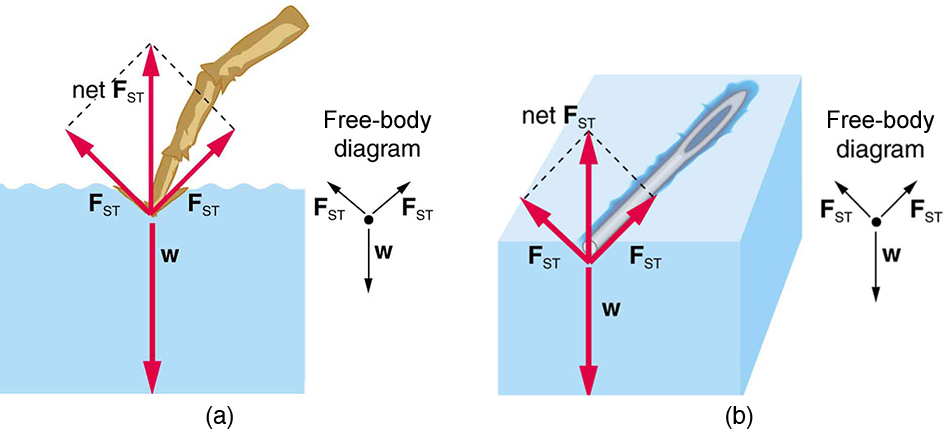
Cohesion And Adhesion In Liquids Surface Tension And Capillary Action Physics
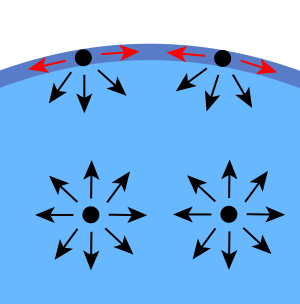
Surface Tension Facts For Kids

Surface Tension And Water

What Is Surface Tension In Hindi Explain Surface Tension In Hindi Define Surface Tension Youtube

3 Ways To Measure Surface Tension Wikihow
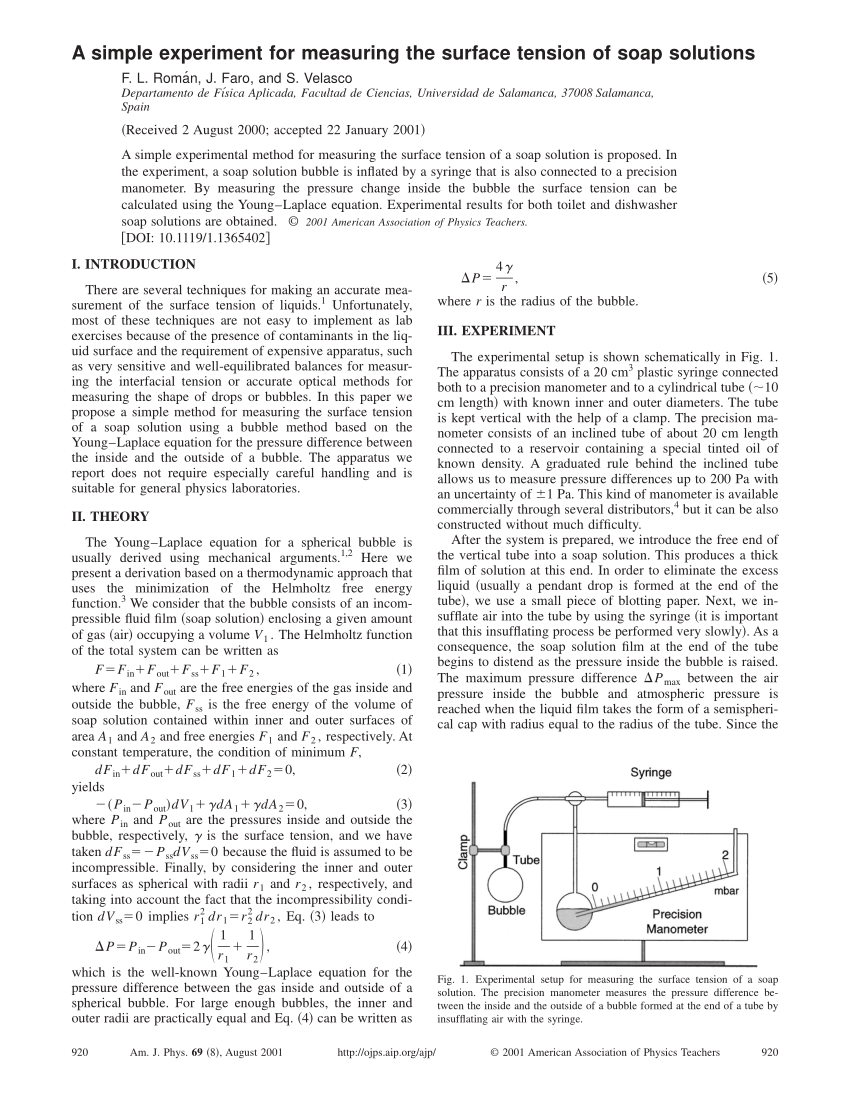
Pdf A Simple Experiment For Measuring The Surface Tension Of Soap Solutions

Surface Tension Definition Examples Facts Britannica
/Surface-Tension-58c6c2365f9b58af5c534f71.jpg)
What Is Surface Tension Definition And Experiments

Surface Tension Wikipedia
Pdf Understanding And Explaining Surface Tension And Capillarity An Introduction To Fundamental Physics For Water Professionals

Surface Tension Basic Physics Tutorials

Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension

11 8 Cohesion And Adhesion In Liquids Surface Tension And Capillary Action College Physics Openstax

A Film Of Water Is Formed Between Two Straight Parallel Wires Each 10 Cm Long And At A Separation Of 0 5 Cm Calculate The Work Required To Increase The Distance Between Them
Arxiv Org Pdf 1708
What Is The Dimensional Formula For Surface Tension Quora
Surface Tension Chemistry Libretexts
Www Utwente Nl En Tnw Pcf Education J M Burgerscentrum Research School For Fluid Mechanics Articles Jacco Snoeijer Snoeijerajp Preprint Pdf

Surface Tension And Adhesion Video Khan Academy

11 8 Cohesion And Adhesion In Liquids Surface Tension And Capillary Action College Physics Openstax

Definition Of Surface Tension Properties Of Fluid Fluid Mechanics Youtube

3 Ways To Measure Surface Tension Wikihow

What Is Surface Tension Wonderopolis

Surface Tension Facts For Kids

Use Surface Tension To Make Pepper Dance Scientific American
Surface Tension And Viscosity Viva Questions Viva Questions And Answers
Www Jstor Org Stable
Http Www Ijcce Ac Ir Article 62 3ab43b165bca41ffea5b14a9d797 Pdf
/139802493-56a12f615f9b58b7d0bcde91.jpg)
Surface Tension Definition In Chemistry

Surface Tension Defintion Experiment Observation Example Youtube