Surface Tension Simple Definition
Surface tension is a property of liquids where the exposed surface shrinks to the sma.

Surface tension simple definition. Surface tension is the tendency of liquids to keep a low surface area For example, if you fill a cup of water to the top, you'll find that the water can actually rise above the lip of the cup slightly The reason it doesn't spill is that the water molecules at the surface hold together like the surface of a balloon, holding the water inside. Definition of surface tension in the Definitionsnet dictionary Meaning of surface tension What does surface tension mean?. What does surfacetension mean?.
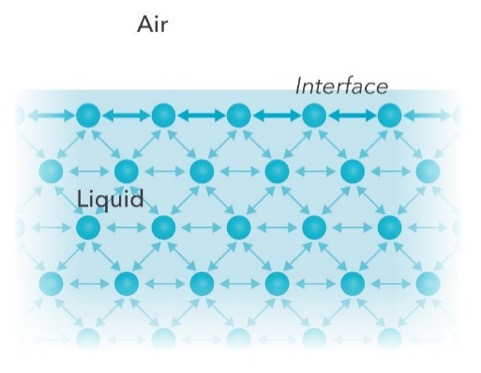
Surface tension is a property of liquids where the exposed surface shrinks to the sma. Surface Tension Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules A molecule in the bulk liquid experiences cohesive forces with other molecules in all directions. Cohesive Force Definition The force of attraction acting between the molecules of same substance is called cohesive force We are giving a detailed and clear sheet on all Physics Notes that are very useful to understand the Basic Physics Concepts Cohesive Force in Physics Definition, Examples – Surface Tension.
Water has high surface tension, which means the molecules are pulling each other on the surface very strongly The pulling is so strong that when you first sprinkle pepper onto the water, it sits on top of the water instead of sinking into it When soap is added in the middle, it breaks the surface tension there but surface tension still exists. What is Surface Tension?. What does surfacetension mean?.
Surface Tension The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension The molecules at the surface do not have other like molecules on all sides of them and consequently they cohere more strongly to those directly associated with them on the surface This forms a surface "film" which makes it more difficult to move an object through the. Tension can be defined as an actionreaction pair of forces acting at each end of the said elements While considering a rope, the tension force is felt by every section of the rope in both the directions, apart from the endpoints The endpoints experience tension on one side and the force from the weight attached. This month’s simple science project will delve into the world of surface tension of water with a couple of simple experiments Surface tension is a barrier formed on the surface of water caused by something called “cohesion” Liquids all have this force, a force that holds a material together Some are stronger than others (liquid mercury.
According to the definition of surface tension, it is the phenomenon that occurs when the surface of a liquid is in contact with another phase (it can be a liquid as well) Liquids tend to acquire the least surface area possible The surface of the liquid behaves like an elastic sheet. Surface tension measurements are done using either force or optical tensiometers Surface tension is an important parameter in many industrial processes which makes its measurement mandatory for process and product optimization. We have observed that, for many different types of liquids, ΔH vap varies linearly with γr c 2 as a function of temperature in the form ΔH vap {T} = κγ c {T}r c 2 {T} κ′ where r c is the radius of molecule C, γ c is the macroscopic surface tension of liquid C and k and k′ are constants independent of temperature To justify physically our empirical observation, a simple model of.
Surface scientists commonly use an optical goniometer/tensiometer to measure the surface tension and interfacial tension of a liquid using the pendant or sessile drop methods A drop is produced and captured using a CCD cameraThe drop profile is subsequently extracted, and sophisticated software routines then fit the theoretical YoungLaplace equation to the experimental drop profile. Define surface tension surface tension synonyms, surface tension pronunciation, surface tension translation, English dictionary definition of surface tension n 1. Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece.
Surface tension definition, the elasticlike force existing in the surface of a body, especially a liquid, tending to minimize the area of the surface, caused by asymmetries in the intermolecular forces between surface molecules See more. Surface tension is the attractive force in liquids that pulls surface molecules into the rest of the liquid, minimizing the surface area These attractive forces are due to electrostatic forces. Here are four surface tension experiments that are supersimple to set up and execute Do all four for a morning full of science & fun!.
Surface tension is the amount of energy required to increase the surface of the liquid by unit area In other words, it is also the property of the liquid surface that resists force Intuitively, it keeps a barrier between foreign materials and liquid as well as this is the force that holds the liquid molecules bind together. Surface tension is an effect where the surface of a liquid is strong The surface can hold up a weight, and the surface of a water droplet holds the droplet together, in a ball shape Some small things can float on a surface because of surface tension, even though they normally could not float Some insects can run on the surface of water because of this This property is caused by the molecules in the liquid being attracted to each other, and is responsible for many of the behaviors of liquids. It has to do with the elastic property of the water surface, a phenomenon called surface tensionSubscribe http//bitl.
Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin It is the high surface tension of water which allows insects to walk over it. Surface tension refers to water molecules that are more closely bound together at the surface, making the top of the water more tight and dense than the rest of the water. The cause of surface tension Even if surface tension is most important for liquids, we shall here consider a simple regular “solid” surrounded by vacuum The molecules are placed in a cubic grid (see the margin figure) with grid length equal to the molecular separation length L mol Each molecule in.
Surface tension depends mainly upon the forces of attraction between the particles within the given liquid and also upon the gas, solid, or liquid in contact with itThe molecules in a drop of water, for example, attract each other weakly Water molecules well inside the drop may be thought of as being attracted equally in all directions by the surrounding molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin It is the high surface tension of water which allows insects to walk over it.
These super simple investigations are great for demonstrating the surface tension of water What is surface tension?. Surface tension is a property of liquids where the exposed surface shrinks to the sma. 1) Introduction to surface tension 2) Surface tension as a line force and interfacial energy 3) Interfacial (liquidliquid) tension 4) Minimal surfaces 5) Soap bubbles, surfactants and detergents 6) Wettability, nonwettability and contact angle hysteresis 7) Role of roughness as an amplifier for wettability.
Magic Finger Surface Tension Experiment. Surface tension 1 n a phenomenon at the surface of a liquid caused by intermolecular forces Types capillarity , capillary action a phenomenon associated with surface tension and resulting in the elevation or depression of liquids in capillaries interfacial surface tension , interfacial tension surface tension at the surface separating two. Simple Surface Tension Experiments Water molecules like to stick together, creating surface tension where the water meets the air This force can be fun to explore!.
(Answer The trapped air is released and the surface tension pulls the water into water droplets) Why does a stream of water form droplets as it falls?. Since surface tension is determined at a molecular level, any change to the component liquids, surfactants, fuels or compounds in a liquid would result in a change in the surface tension If the surface tension of a perfectly pure composition is known, any variation from that would reveal some level of contamination. Surface Tension Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules A molecule in the bulk liquid experiences cohesive forces with other molecules in all directions.
Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet This term is typically used only when the liquid surface is in contact with gas (such as the air) If the surface is between two liquids (such as water and oil), it is called "interface tension". Surface tension tends to reduce with increasing temperature As a liquid heats up, the molecules in it speed up, which tends to break the bonds produced by cohesive forces Table 2 shows the effect of temperature on the surface tension of water The colder the water, the higher the surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties.
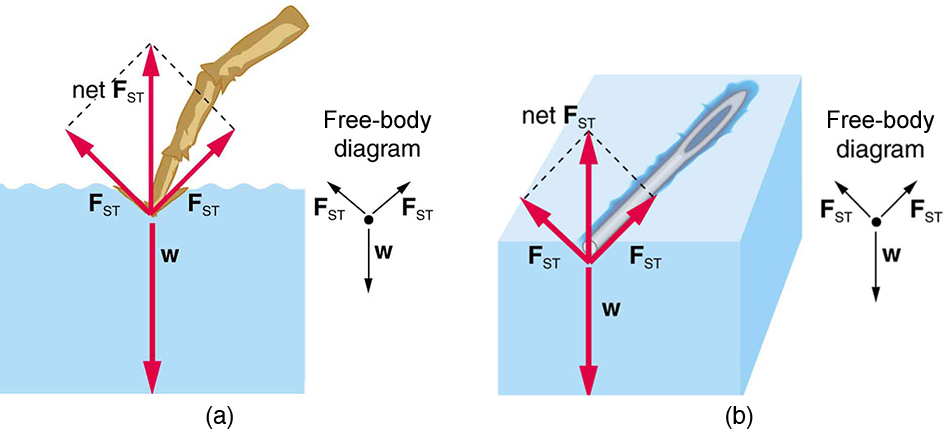
How do water striders walk on water?. Surface tension allows objects that are denser than water, such as the paper clip shown in B in Figure below , to nonetheless float on its surface It is also responsible for the beading up of water droplets on a freshly waxed car because there are no attractions between the polar water molecules and the nonpolar wax. Information and translations of surface tension in the most comprehensive dictionary definitions resource on the web.
The definition of Liquid Surface Tension is simple It's the force that keeps a liquid from flying off into space However, the measurement of surface tension can take many forms, which can be confusing. Surface Tension In Physics, the tension of the surface film of a liquid because of the attraction of the surface particles by the bulk of the liquid, which tries to minimize surface area is called surface tension When the surface of the liquid is strong enough, then surface tension is applicable It is strong enough to hold weight. It is caused by unbalanced intermolecular forces.
The cause of surface tension Even if surface tension is most important for liquids, we shall here consider a simple regular “solid” surrounded by vacuum The molecules are placed in a cubic grid (see the margin figure) with grid length equal to the molecular separation length L mol Each molecule in. For the simple case of a free surface (such as airwater, in which one of the fluids is not dynamically significant) in order to get a feeling for the scaling of surface tension The normal stress balance at a free surface must be balanced by the curvature force associated with the surface tension n·T·n = σ (∇·n) (3). What does surfacetension mean?.
Surface tension Wikipedia A coin suspended on water by surface tension Noun surface tension (countable and uncountable, plural surface tensions) the effect on the surface of a liquid that makes it behave as a stretched elastic membrane;. Surface tension definition is the attractive force exerted upon the surface molecules of a liquid by the molecules beneath that tends to draw the surface molecules into the bulk of the liquid and makes the liquid assume the shape having the least surface area. Factors affecting Surface Tension The value of surface tension for a particular liquid can be affected by the nature of the liquid, surroundings and purity of the liquid Let’s discuss these factors in a detailed manner 1 Temperature The surface tension of liquid decreases with an increase in temperature.
These super simple investigations are great for demonstrating the surface tension of water What is surface tension?. The surface tension tries to minimize the surface area For the same volume of air, a sphere has a smaller surface area than a cube) What happens when a soap bubble pops?. Surface tension is the tensile force acting on either side of any imaginary line projected on the surface of a liquidit can be also defined by energy per unit area Surface tension develop only at the surface of liquid not inside itbecause of.
Factors affecting Surface Tension The value of surface tension for a particular liquid can be affected by the nature of the liquid, surroundings and purity of the liquid Let’s discuss these factors in a detailed manner 1 Temperature The surface tension of liquid decreases with an increase in temperature. Units of Surface Tension Surface tension is expressed in units of force per unit length or of energy per unit area (for instance, N/m or J/m 2) The two are equivalent, but when referring to energy per unit area, people use the term “surface energy,” which is a more general term in the sense that it applies to solids as well as to liquids. 2) Mechanical definition of surface tension Thus surface tension can manifest itself both in forms of surface energy as well as surface force (i) as an energy necessary to create surfaces Work is needed to increase the surface area eg you perform 'work' when you beat egg whites into a meringue, or you make an emulsion of water in oil while.
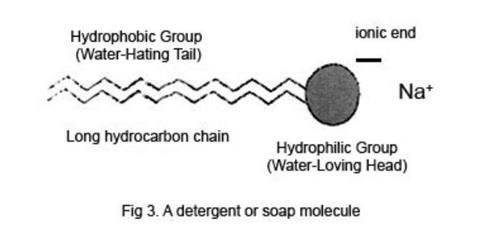
Surface tension is also defined as the tensile forces acting on the surface of a liquid in contact with a gas or on the surface between two immiscible liquids such that the contact surface behaves like a membrane under tension. Surface tension disinfectants Disinfectants are usually solutions of low surface tension This allow them to spread out on the cell walls of bacteria and disrupt them Soaps and detergents These help the cleaning of clothes by lowering the surface tension of the water so that it more readily soaks into pores and soiled areas. To determine the surface tension γγγγ the Wilhelmy equation is applied If the plate has a width l and its weight is W plate, then the force F needed to detach it from the liquid surface equals F = W total = W plate 2 l γγγγ cos θθθθ (16) Multiplying by 2 is needed because the surface tension acts on both sides of the plate,.
Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane This phenomenon can be observed in the nearly spherical shape of small drops of liquids and of soap bubbles Because of this property, certain insects can stand on the surface of water. For the simple case of a free surface (such as airwater, in which one of the fluids is not dynamically significant) in order to get a feeling for the scaling of surface tension The normal stress balance at a free surface must be balanced by the curvature force associated with the surface tension n·T·n = σ (∇·n) (3). Surface tension of mixed nonionic surfactant/protein solutions comparison of a simple theoretical model with experiments Colloids and Surfaces A Physicochemical and Engineering Aspects 04, 233 (13) , 3942 DOI /jcolsurfa.
Surface tension definition a property of liquids caused by intermolecular forces near the surface leading to the Meaning, pronunciation, translations and examples. Simply put, surface tension is the force behind the film formed by the surface of a liquid For example, imagine a water drop sitting on a flat piece.

Surface Energy And Angle Of Contact Youtube
Surface And Interfacial Tension Nanoscience Instruments
Detergents Soaps And Surface Tension Experiment Rsc Education
Surface Tension Simple Definition のギャラリー

Surface Tension Simple English Wikipedia The Free Encyclopedia
Cohesion And Adhesion In Liquids Surface Tension And Capillary Action Physics

What Is The Difference Between Surface Tension And Surface Energy Quora

Investigating The Impact Of Sugar Based Surfactants Structure On Surface Tension At Critical Micelle Concentration With Structure Property Relationships Sciencedirect

Capillary Action Wikipedia

Surface Tension What Is Surface Tension Kibron
:max_bytes(150000):strip_icc()/139802493-56a12f615f9b58b7d0bcde91.jpg)
Surface Tension Definition In Chemistry

Surface Tension And Water

What Is Surface Tension
What Is The Difference Between Surface And Interfacial Tension

Surface Tension Simple English Wikipedia The Free Encyclopedia

Simple Definations Surface Tension
What Is The Difference Between Surface Tension And Surface Energy Quora
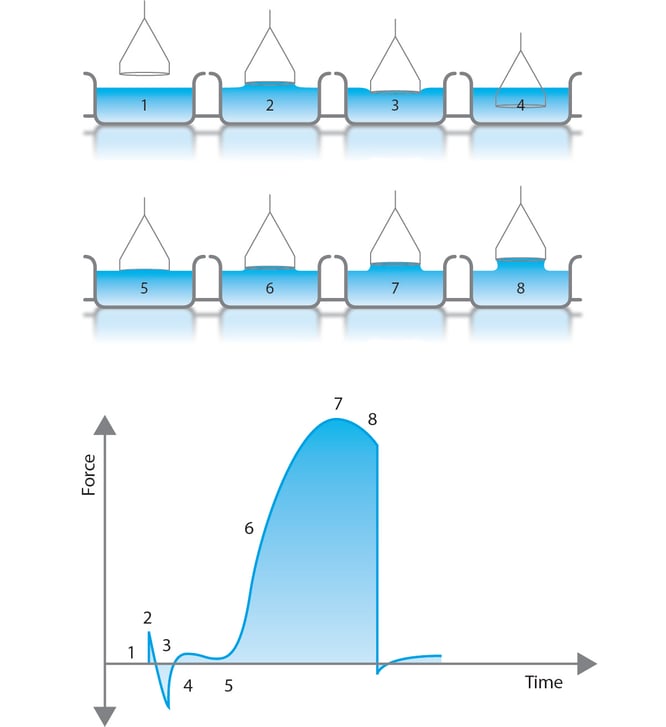
3 Ways To Measure Surface Tension

Surface Tension And Water
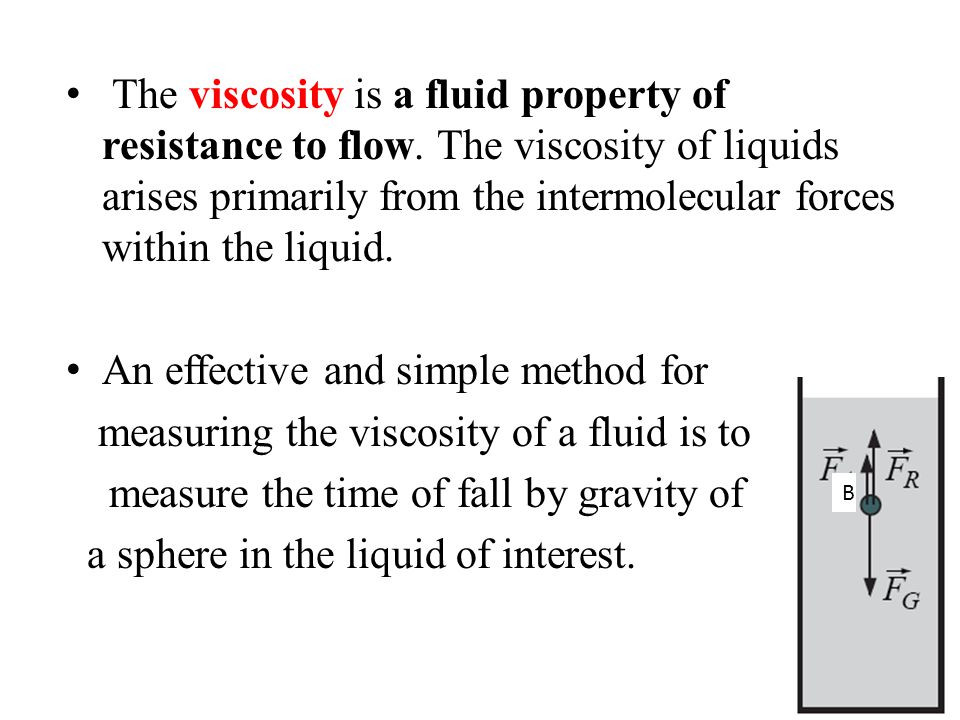
Lecture 7 Flow Of Ideal Liquid Viscosity Diffusion Surface Tension Ppt Video Online Download

Surface Tension Simple English Wikipedia The Free Encyclopedia

What Are Cohesive And Adhesive Forces Define The Term Surface Tension And Give Its Si Unit Quora

Surface Tension Formula Definition Equations Examples
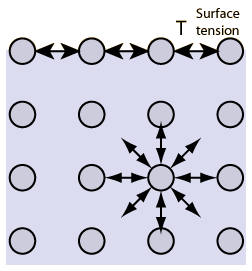
Surface Tension
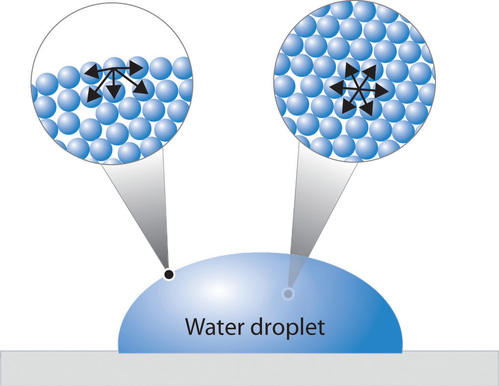
Surface Tension Chemistry Libretexts
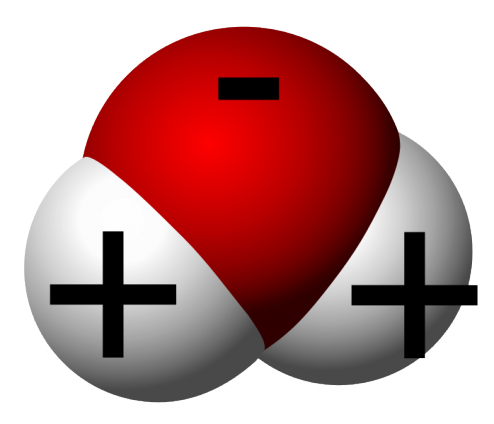
Surface Tension Archives Adib Behjat

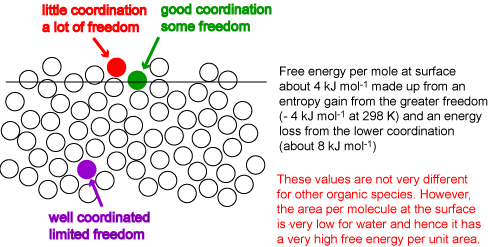
The Science Of New Wash Science Physical Properties Phase Rule

Science Experiments For Kids Exploring Surface Tension Buggy And Buddy

What Is Surface Tension

Surface Tension What Is Surface Tension Kibron

Surface Tension Ideas Surface Tension Tension Science Experiments

Capillary Action Of Water Definition Examples Video Lesson Transcript Study Com

How Does Soap Work Ida S Soap Box
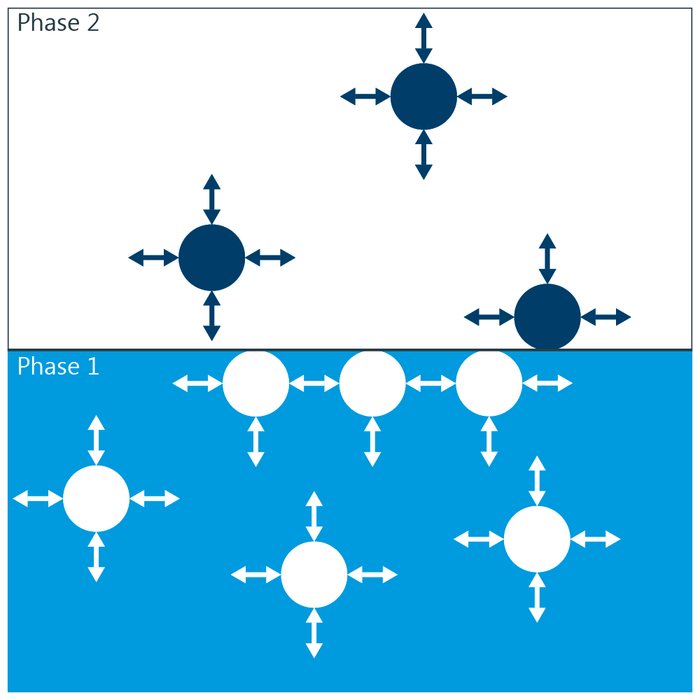
The Thomas Group Ptcl Oxford

Sounds Like Surface Tension Imagination Station

Tension Definition Explanation Solved Problems Faqs

Surface Tension Surface Energy Dataphysics Instruments

Water And Its Structure

Definition Of Surface Tension Properties Of Fluid Fluid Mechanics Youtube
Surface Tension Kruss Scientific

Surface Tension Defintion Experiment Observation Example Youtube

Lung Alveolus Surface Tension An Overview Sciencedirect Topics

Surface Tension And Water
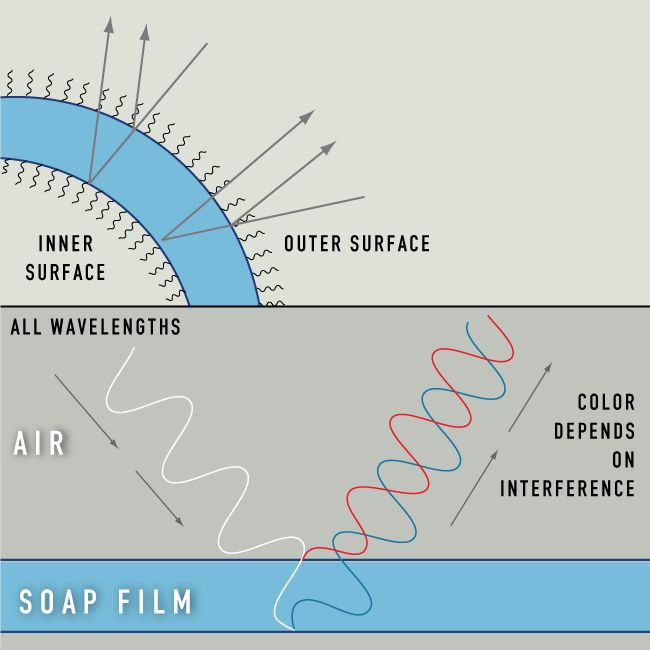
Bubbles Causes Of Color

Science Experiments For Kids Exploring Surface Tension Buggy And Buddy
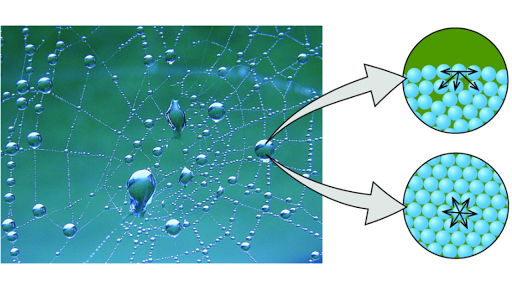
Cohesion And Adhesion Of Water Article Khan Academy

10 Best Surface Tension Ideas Surface Tension Tension Surface

What Is Surface Energy Se Youtube

Surface Tension

Surface Tension And Water

Surface Tension Defintion Experiment Observation Example Youtube

What Is Surface Tension Kids Science Experiments Youtube
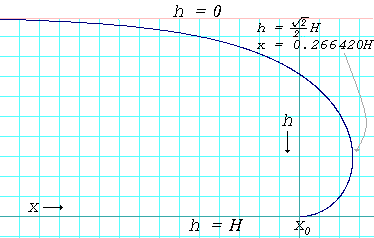
Surface Tension Simple English Wikipedia The Free Encyclopedia

Viscosity Surface Tension Definition With Examples Of Viscosity Surface Tension
/Surface-Tension-58c6c2365f9b58af5c534f71.jpg)
What Is Surface Tension Definition And Experiments

Physics Free Notes Surface Tension Theory By Atc

Surface Tension Measurements

Surfactant Definition Properties Examples Facts Britannica

Surface Tension Definition Causes Measurement Formula Video Lesson Transcript Study Com

Surface Tension What Is Surface Tension Kibron
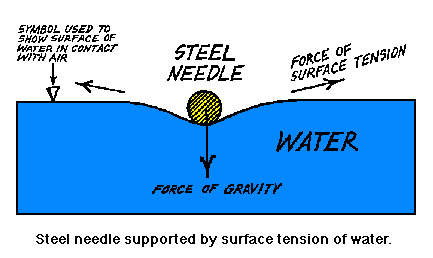
Structure And Properties Of Water

Surface Tension Wikipedia

Surface Tension Definition Examples Facts Britannica
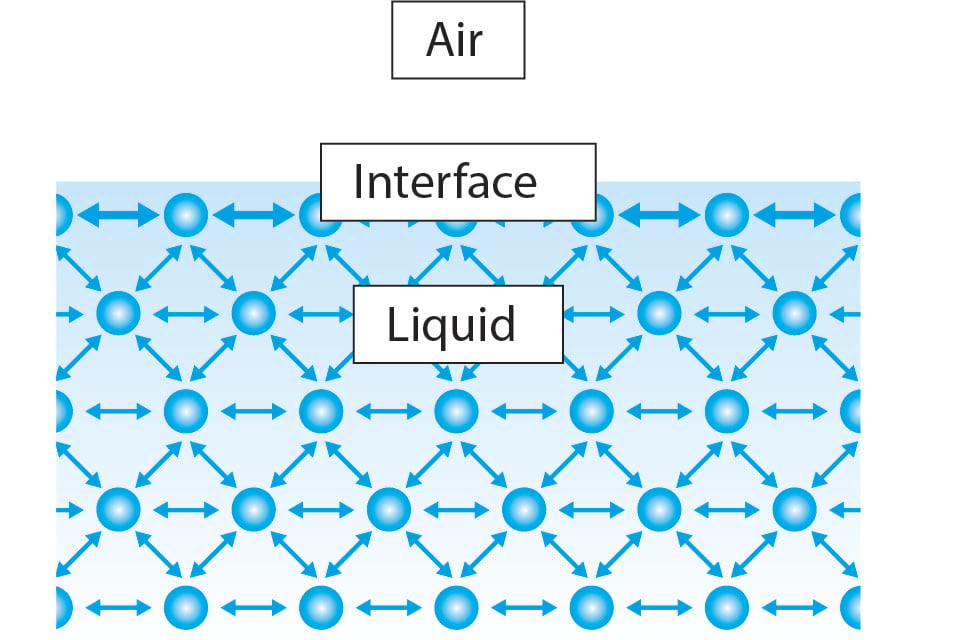
Surface Tension Of Water Why Is It So High

Surface Tension Simple English Wikipedia The Free Encyclopedia
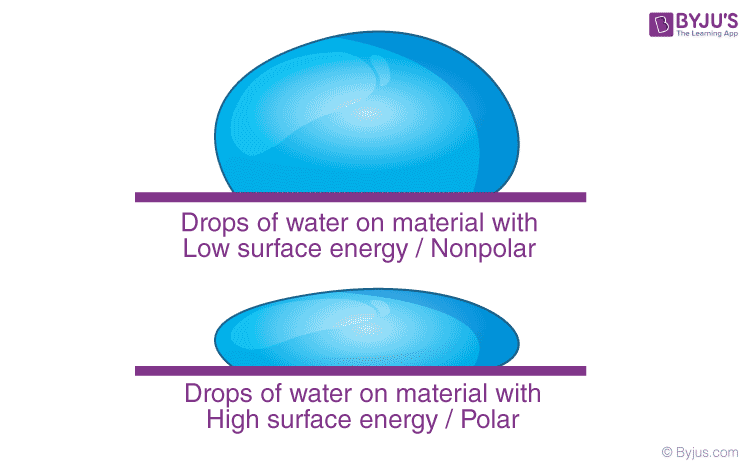
What Is Surface Energy Definition Formula Units Equation

What Is Surface Tension Definition Si Unit Formula Dimension And Examples

Surface Tension Introduction To Chemistry
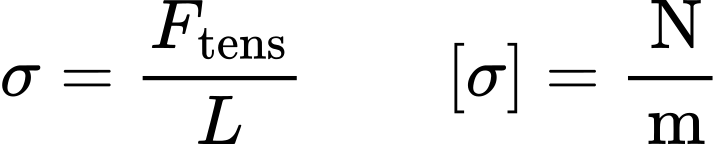
Surface Tension Surface Energy Dataphysics Instruments

Surface Tension Physics Made Easy Facebook

10 Best Surface Tension Ideas Surface Tension Tension Surface

Surface Tension Definition Examples Facts Britannica

Surface Tension Video Chemistry Of Life Khan Academy
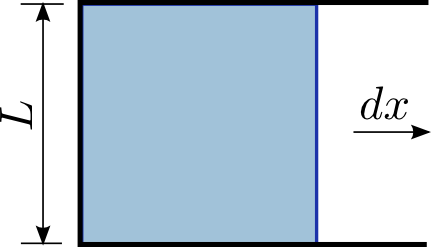
Surface Tension Facts For Kids

Definition Of Surface Tension Properties Of Fluid Fluid Mechanics Youtube

Surface Tension

Surface Tension Definition Examples Facts Britannica

Measurement Of Surface And Interfacial Tension Using Pendant Drop Tensiometry Sciencedirect

Forces And Motion Forces For Kids Physical Processes For Kids Force And Motion Gravity Lessons Motion

Surface Tension Grade 2 5
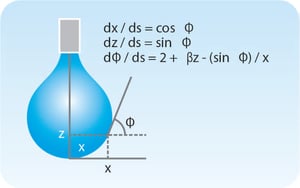
Surface Tension Measurements

Surface Energy Wikipedia

Surface And Interfacial Tension
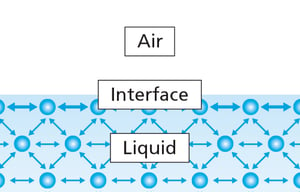
Surface Tension Measurements
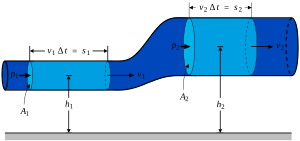
Surface Tension Facts For Kids
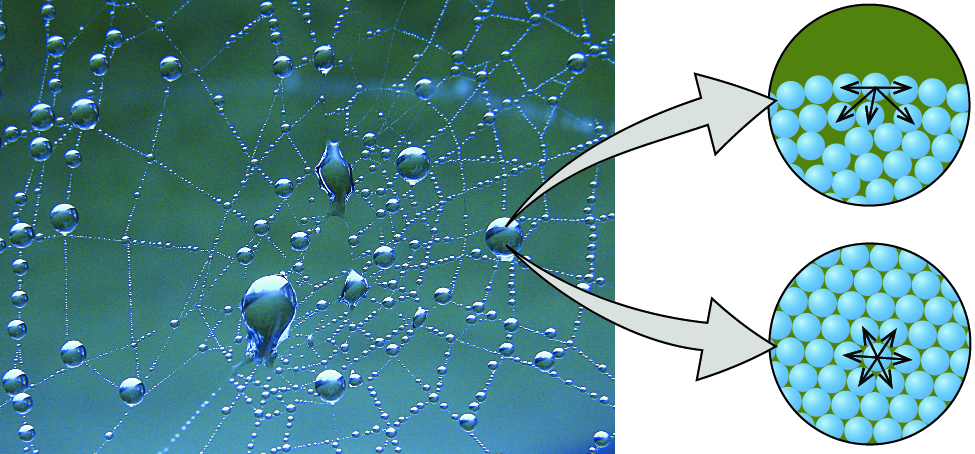
Cohesion And Adhesion Of Water Article Khan Academy
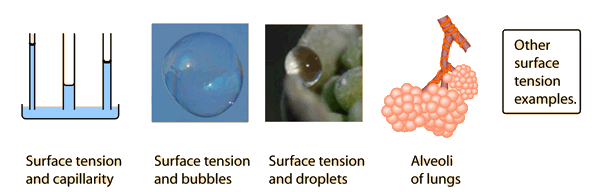
Surface Tension
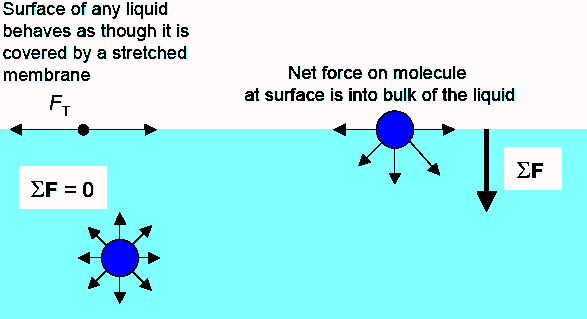
Surface Tension
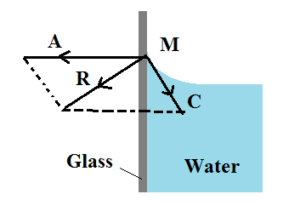
Angle Of Contact Meaning Its Characteristics And Significance
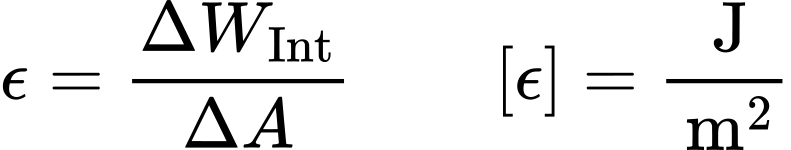
Surface Tension Surface Energy Dataphysics Instruments
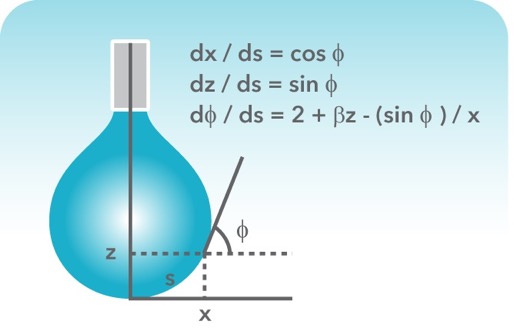
Surface And Interfacial Tension Nanoscience Instruments

Surface Tension And Water

Surface Tension Simple English Wikipedia The Free Encyclopedia
What Is The Difference Between Surface Tension And Surface Energy Quora

Surface Tension Surface Energy Dataphysics Instruments

Surface Tension On Liquid Droplet Basic Of Fluid Mechanics 22 Anuniverse 22 Youtube

Rame Hart Instrument Co Monthly Newsletter

What Is Surface Energy Calculation Models And More Explained Ossila
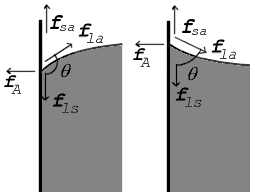
Surface Tension Simple English Wikipedia The Free Encyclopedia

Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube